Disparate adjuvant properties among three formulations of "alum"
- PMID: 23200935
- PMCID: PMC3541451
- DOI: 10.1016/j.vaccine.2012.11.044
Disparate adjuvant properties among three formulations of "alum"
Abstract
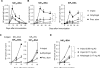
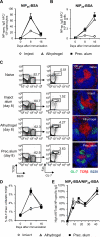
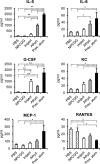
Aluminum adjuvants, commonly referred to as "alum," are the most widespread immunostimulants in human vaccines. Although the mechanisms that promote humoral responses to alum-adsorbed antigens are still enigmatic, alum is thought to form antigen depots and induce inflammatory signals that, in turn, promote antibody production. It was recently noted that Imject(®) alum, a commercial aluminum-containing adjuvant commonly used in animal studies, is not the physicochemical equivalent of aluminum adjuvant present in human vaccines. This difference raises concerns about the use of Imject(®) alum in animal research as a model for approved aluminum adjuvants. Here, we compared the capacity of Imject(®) alum, Alhydrogel(®), and a traditional alum-antigen precipitate to induce humoral responses in mice to the hapten-carrier antigen, NP-CGG [(4-hydroxy-3-nitrophenyl)acetyl-chicken γ-globulin]. The magnitude of humoral responses elicited by Alhydrogel(®) and precipitated alum was significantly greater than that induced by Imject(®) alum. The strength of the humoral responses elicited by different alum formulations was correlated with the quantity of pro-inflammatory cytokines induced and the numbers of inflammatory cells at the site of immunization. Moreover, Imject(®) exhibited a severely reduced capacity to adsorb protein antigens compared to Alhydrogel(®) and precipitated alum. These findings reveal substantial differences in the immunostimulatory properties of distinct alum preparations, an important point of consideration for the evaluation of novel adjuvants, the assessment of new alum-based vaccines, and in mechanistic studies of adjuvanticity.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine. 2010;28(Suppl 3):C25–36. - PubMed
-
- Vogel FR, Powell MF. A compendium of vaccine adjuvants and excipients. Pharm Biotechnol. 1995;6:141–228. - PubMed
-
- Shirodkar S, et al. Aluminum compounds used as adjuvants in vaccines. Pharm Res. 1990;7(12):1282–8. - PubMed
-
- Edelman R. Vaccine adjuvants. Rev Infect Dis. 1980;2(3):370–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

