Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus
- PMID: 23201030
- PMCID: PMC4191647
- DOI: 10.1016/j.heares.2012.11.016
Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus
Abstract
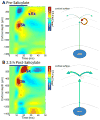
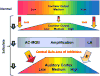
High doses of sodium salicylate (SS) have long been known to induce temporary hearing loss and tinnitus, effects attributed to cochlear dysfunction. However, our recent publications reviewed here show that SS can induce profound, permanent, and unexpected changes in the cochlea and central nervous system. Prolonged treatment with SS permanently decreased the cochlear compound action potential (CAP) amplitude in vivo. In vitro, high dose SS resulted in a permanent loss of spiral ganglion neurons and nerve fibers, but did not damage hair cells. Acute treatment with high-dose SS produced a frequency-dependent decrease in the amplitude of distortion product otoacoustic emissions and CAP. Losses were greatest at low and high frequencies, but least at the mid-frequencies (10-20 kHz), the mid-frequency band that corresponds to the tinnitus pitch measured behaviorally. In the auditory cortex, medial geniculate body and amygdala, high-dose SS enhanced sound-evoked neural responses at high stimulus levels, but it suppressed activity at low intensities and elevated response threshold. When SS was applied directly to the auditory cortex or amygdala, it only enhanced sound evoked activity, but did not elevate response threshold. Current source density analysis revealed enhanced current flow into the supragranular layer of auditory cortex following systemic SS treatment. Systemic SS treatment also altered tuning in auditory cortex and amygdala; low frequency and high frequency multiunit clusters up-shifted or down-shifted their characteristic frequency into the 10-20 kHz range thereby altering auditory cortex tonotopy and enhancing neural activity at mid-frequencies corresponding to the tinnitus pitch. These results suggest that SS-induced hyperactivity in auditory cortex originates in the central nervous system, that the amygdala potentiates these effects and that the SS-induced tonotopic shifts in auditory cortex, the putative neural correlate of tinnitus, arises from the interaction between the frequency-dependent losses in the cochlea and hyperactivity in the central nervous system.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures







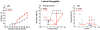



References
-
- Akada S, Takeda S, Ogawa R. Salicylate action on medullary inspiratory neuron activity in a brainstem-spinal cord preparation from newborn rats. Anesth Analg. 2003;96:407–11. table of contents. - PubMed
-
- Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound--questionnaire data, audiometry and classification. Scand Audiol. 1999;28:219–30. - PubMed
-
- Andersson G, Lindvall N, Hursti T, Carlbring P. Hypersensitivity to sound (hyperacusis): a prevalence study conducted via the Internet and post. Int J Audiol. 2002;41:545–54. - PubMed
-
- Bauer CA, Brozoski TJ, Holder TM, Caspary DM. Effects of chronic salicylate on GABAergic activity in rat inferior colliculus. Hear Res. 2000;147:175–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

