Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis
- PMID: 23201165
- PMCID: PMC3522773
- DOI: 10.1016/j.ccr.2012.09.022
Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis
Abstract
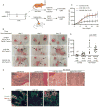
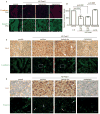
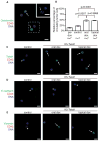
Epithelial-mesenchymal transition (EMT) is implicated in converting stationary epithelial tumor cells into motile mesenchymal cells during metastasis. However, the involvement of EMT in metastasis is still controversial, due to the lack of a mesenchymal phenotype in human carcinoma metastases. Using a spontaneous squamous cell carcinoma mouse model, we show that activation of the EMT-inducing transcription factor Twist1 is sufficient to promote carcinoma cells to undergo EMT and disseminate into blood circulation. Importantly, in distant sites, turning off Twist1 to allow reversion of EMT is essential for disseminated tumor cells to proliferate and form metastases. Our study demonstrates in vivo the requirement of "reversible EMT" in tumor metastasis and may resolve the controversy on the importance of EMT in carcinoma metastasis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Metastasis: Epithelial to mesenchymal and back again.Nat Rev Cancer. 2013 Jan;13(1):3. doi: 10.1038/nrc3428. Nat Rev Cancer. 2013. PMID: 23258155 No abstract available.
References
-
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. - PubMed
-
- Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis. 2006;44:23–28. - PubMed
-
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. - PubMed
-
- Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

