VMAT1 deletion causes neuronal loss in the hippocampus and neurocognitive deficits in spatial discrimination
- PMID: 23201251
- PMCID: PMC4364608
- DOI: 10.1016/j.neuroscience.2012.11.023
VMAT1 deletion causes neuronal loss in the hippocampus and neurocognitive deficits in spatial discrimination
Abstract
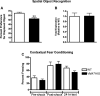
Vesicular monoamine transporters (VMAT) are involved in presynaptic storage and release of neurotransmitters. While it was thought initially that only VMAT2 is brain expressed and VMAT1 is present only in the periphery, recent data have challenged the exclusive expression of VMAT2 in the brain. To further elucidate the role of VMAT1 brain expression and its potential role in neuropsychiatric disorders, we have investigated mice lacking VMAT1. Comparison of wildtype and knock-out (KO) mice using qPCR and immunohistochemistry documents the expression of VMAT1 in the brain. Deletion of VMAT1 leads to increased hippocampal apoptosis and reduced neurogenesis as assessed by caspase-3-labeling and 5-bromo-deoxy-uridine-labeling. Behavioral data show that mice lacking VMAT1 have neurocognitive deficits. VMAT2 expression is not altered in VMAT1 KO mice, suggesting a distinct role of VMAT1. Our data support VMAT1 brain expression and suggest that VMAT1 plays a key role in survival of hippocampal neurons and thus might contribute to neurocognitive deficits observed in neuropsychiatric disorders.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures







Similar articles
-
Deletion of the vesicular monoamine transporter 1 (vmat1/slc18a1) gene affects dopamine signaling.Brain Res. 2019 Jun 1;1712:151-157. doi: 10.1016/j.brainres.2019.01.029. Epub 2019 Jan 24. Brain Res. 2019. PMID: 30685272
-
Vesicular monoamine transporter protein expression correlates with clinical features, tumor biology, and MIBG avidity in neuroblastoma: a report from the Children's Oncology Group.Eur J Nucl Med Mol Imaging. 2016 Mar;43(3):474-481. doi: 10.1007/s00259-015-3179-2. Epub 2015 Sep 4. Eur J Nucl Med Mol Imaging. 2016. PMID: 26338179 Free PMC article.
-
Genetic variants in the vesicular monoamine transporter 1 (VMAT1/SLC18A1) and neuropsychiatric disorders.Methods Mol Biol. 2010;637:165-80. doi: 10.1007/978-1-60761-700-6_9. Methods Mol Biol. 2010. PMID: 20419435
-
[Involvement of vesicular monoamine transporter in attention deficit hyperactivity disorder].Rev Neurol. 2011 Mar 1;52 Suppl 1:S103-8. Rev Neurol. 2011. PMID: 21365590 Review. Spanish.
-
Vesicular monoamine transporter (VMAT) regional expression and roles in pathological conditions.Heliyon. 2023 Nov 15;9(11):e22413. doi: 10.1016/j.heliyon.2023.e22413. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034713 Free PMC article. Review.
Cited by
-
Activating adenosine A1 receptor accelerates PC12 cell injury via ADORA1/PKC/KATP pathway after intermittent hypoxia exposure.Mol Cell Biochem. 2018 Sep;446(1-2):161-170. doi: 10.1007/s11010-018-3283-2. Epub 2018 Jan 29. Mol Cell Biochem. 2018. PMID: 29380238
-
Resting-state functional connectivity and presynaptic monoamine signaling in Alcohol Dependence.Hum Brain Mapp. 2015 Dec;36(12):4808-18. doi: 10.1002/hbm.22951. Epub 2015 Sep 14. Hum Brain Mapp. 2015. PMID: 26368063 Free PMC article.
-
Humanized substitutions of Vmat1 in mice alter amygdala-dependent behaviors associated with the evolution of anxiety.iScience. 2022 Jul 20;25(8):104800. doi: 10.1016/j.isci.2022.104800. eCollection 2022 Aug 19. iScience. 2022. PMID: 35992083 Free PMC article.
-
Protective effect of bone marrow mesenchymal stem cells on PC12 cells apoptosis mediated by TAG1.Int J Clin Exp Pathol. 2015 Oct 1;8(10):12093-100. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26722394 Free PMC article.
-
Functional and Biochemical Consequences of Disease Variants in Neurotransmitter Transporters: A Special Emphasis on Folding and Trafficking Deficits.Pharmacol Ther. 2021 Jun;222:107785. doi: 10.1016/j.pharmthera.2020.107785. Epub 2020 Dec 10. Pharmacol Ther. 2021. PMID: 33310157 Free PMC article. Review.
References
-
- Ashe KM, Chiu WL, Khalifa AM, Nicolas AN, Brown BL, De Martino RR, Alexander CP, Waggener CT, Fischer-Stenger K, Stewart JK. Vesicular monoamine transporter-1 (VMAT-1) mRNA and immunoreactive proteins in mouse brain. Neuro Endocrinol Lett. 2011;32:253–258. - PubMed
-
- Balu DT, Hodes GE, Hill TE, Ho N, Rahman Z, Bender CN, Ring RH, Dwyer JM, Rosenzweig-Lipson S, Hughes ZA, Schechter LE, Lucki I. Flow cytometric analysis of BrdU incorporation as a high-throughput method for measuring adult neurogenesis in the mouse. J Pharmacol Toxicol Methods. 2009;59:100–107. - PMC - PubMed
-
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biological psychiatry. 1998;44:88–97. - PubMed
-
- Bly M. Mutation in the vesicular monoamine gene, SLC18A1, associated with schizophrenia. Schizophrenia research. 2005;78:337–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

