Viral models of multiple sclerosis: neurodegeneration and demyelination in mice infected with Theiler's virus
- PMID: 23201558
- PMCID: PMC7117056
- DOI: 10.1016/j.pneurobio.2012.11.003
Viral models of multiple sclerosis: neurodegeneration and demyelination in mice infected with Theiler's virus
Abstract
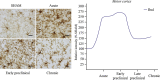
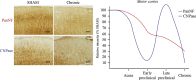
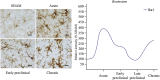
Multiple sclerosis (MS) is a complex inflammatory disease of unknown etiology that affects the central nervous system (CNS) white matter, and for which no effective cure exists. Indeed, whether the primary event in MS pathology affects myelin or axons of the CNS remains unclear. Animal models are necessary to identify the immunopathological mechanisms involved in MS and to develop novel therapeutic and reparative approaches. Specifically, viral models of chronic demyelination and axonal damage have been used to study the contribution of viruses in human MS, and they have led to important breakthroughs in our understanding of MS pathology. The Theiler's murine encephalomyelitis virus (TMEV) model is one of the most commonly used MS models, although other viral models are also used, including neurotropic strains of mouse hepatitis virus (MHV) that induce chronic inflammatory demyelination with similar histological features to those observed in MS. This review will discuss the immunopathological mechanisms involved in TMEV-induced demyelinating disease (TMEV-IDD). The TMEV model reproduces a chronic progressive disease due to the persistence of the virus for the entire lifespan in susceptible mice. The evolution and significance of the axonal damage and neuroinflammation, the importance of epitope spread from viral to myelin epitopes, the presence of abortive remyelination and the existence of a brain pathology in addition to the classical spinal cord demyelination, are some of the findings that will be discussed in the context of this TMEV-IDD model. Despite their limitations, viral models remain an important tool to study the etiology of MS, and to understand the clinical and pathological variability associated with this disease.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


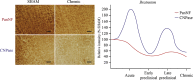
Similar articles
-
Comparison of Reported Spinal Cord Lesions in Progressive Multiple Sclerosis with Theiler's Murine Encephalomyelitis Virus Induced Demyelinating Disease.Int J Mol Sci. 2019 Feb 25;20(4):989. doi: 10.3390/ijms20040989. Int J Mol Sci. 2019. PMID: 30823515 Free PMC article.
-
Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler's virus-infected mice.J Clin Invest. 1999 Sep;104(5):599-610. doi: 10.1172/JCI7292. J Clin Invest. 1999. PMID: 10487774 Free PMC article.
-
Neuropathogenesis of Theiler's murine encephalomyelitis virus infection, an animal model for multiple sclerosis.J Neuroimmune Pharmacol. 2010 Sep;5(3):355-69. doi: 10.1007/s11481-009-9179-x. Epub 2009 Nov 6. J Neuroimmune Pharmacol. 2010. PMID: 19894121 Free PMC article. Review.
-
Two models of multiple sclerosis: experimental allergic encephalomyelitis (EAE) and Theiler's murine encephalomyelitis virus (TMEV) infection. A pathological and immunological comparison.Microsc Res Tech. 1995 Oct 15;32(3):215-29. doi: 10.1002/jemt.1070320305. Microsc Res Tech. 1995. PMID: 8527856 Free PMC article. Review.
-
Resveratrol exacerbates both autoimmune and viral models of multiple sclerosis.Am J Pathol. 2013 Nov;183(5):1390-1396. doi: 10.1016/j.ajpath.2013.07.006. Epub 2013 Oct 1. Am J Pathol. 2013. PMID: 24091251 Free PMC article.
Cited by
-
A monoclonal natural human IgM protects axons in the absence of remyelination.J Neuroinflammation. 2016 Apr 28;13(1):94. doi: 10.1186/s12974-016-0561-3. J Neuroinflammation. 2016. PMID: 27126523 Free PMC article.
-
Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis.PLoS One. 2018 Sep 19;13(9):e0202590. doi: 10.1371/journal.pone.0202590. eCollection 2018. PLoS One. 2018. PMID: 30231069 Free PMC article.
-
Comparison of Reported Spinal Cord Lesions in Progressive Multiple Sclerosis with Theiler's Murine Encephalomyelitis Virus Induced Demyelinating Disease.Int J Mol Sci. 2019 Feb 25;20(4):989. doi: 10.3390/ijms20040989. Int J Mol Sci. 2019. PMID: 30823515 Free PMC article.
-
Synaptic plasticity in multiple sclerosis and in experimental autoimmune encephalomyelitis.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 2;369(1633):20130162. doi: 10.1098/rstb.2013.0162. Print 2014 Jan 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24298163 Free PMC article. Review.
-
Social disruption alters pain and cognition in an animal model of multiple sclerosis.J Neuroimmunol. 2015 Nov 15;288:56-68. doi: 10.1016/j.jneuroim.2015.09.005. Epub 2015 Sep 12. J Neuroimmunol. 2015. PMID: 26531695 Free PMC article.
References
-
- Ahlgren C., Odén A., Berströmg T., Lycke J. Serum and CSF measles antibody levels increase over time in multiple sclerosis or clinically isolated syndrome. Journal of Neuroimmunology. 2012;247:70–74. - PubMed
-
- Allen I., Brankin B. Pathogenesis of multiple sclerosis: the immune diathesis and the role of viruses. Journal of Neuropathology and Experimental Neurology. 1993;52:95–105. - PubMed
-
- Amor S., Scallan M.F., Morris M.M., Dyson H., Fazakerley J.K. Role of immune responses in protection and pathogenesis during Semliki Forest virus encephalitis. Journal of General Virology. 1996;77:281–291. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

