Practical and innate carbon-hydrogen functionalization of heterocycles
- PMID: 23201691
- PMCID: PMC3518649
- DOI: 10.1038/nature11680
Practical and innate carbon-hydrogen functionalization of heterocycles
Abstract
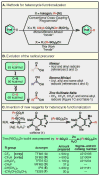
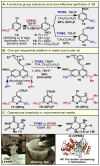
Nitrogen-rich heterocyclic compounds have had a profound effect on human health because these chemical motifs are found in a large number of drugs used to combat a broad range of diseases and pathophysiological conditions. Advances in transition-metal-mediated cross-coupling have simplified the synthesis of such molecules; however, C-H functionalization of medicinally important heterocycles that does not rely on pre-functionalized starting materials is an underdeveloped area. Unfortunately, the innate properties of heterocycles that make them so desirable for biological applications--such as aqueous solubility and their ability to act as ligands--render them challenging substrates for direct chemical functionalization. Here we report that zinc sulphinate salts can be used to transfer alkyl radicals to heterocycles, allowing for the mild (moderate temperature, 50 °C or less), direct and operationally simple formation of medicinally relevant C-C bonds while reacting in a complementary fashion to other innate C-H functionalization methods (Minisci, borono-Minisci, electrophilic aromatic substitution, transition-metal-mediated C-H insertion and C-H deprotonation). We prepared a toolkit of these reagents and studied their reactivity across a wide range of heterocycles (natural products, drugs and building blocks) without recourse to protecting-group chemistry. The reagents can even be used in tandem fashion in a single pot in the presence of water and air.
Conflict of interest statement
The authors declare no competing financial interests. Supplementary Information and chemical compound information accompany this paper at
Figures
Comment in
-
Organic chemistry: Toolkit of reagents to aid drug discovery.Nature. 2012 Dec 6;492(7427):45-6. doi: 10.1038/nature11760. Epub 2012 Nov 28. Nature. 2012. PMID: 23201687 No abstract available.
References
-
- Minisci F, Vismara E, Fontana F. Recent developments of free-radical substitutions of heteroaromatic bases. Heterocycles. 1989;28:489–519.
-
- Duncton MAJ. Minisci reactions: versatile CH-functionalizations for medicinal chemists. Med Chem Comm. 2011;2:1135–1161.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

