MicroRNA-21 silencing enhances the cytotoxic effect of the antiangiogenic drug sunitinib in glioblastoma
- PMID: 23201752
- PMCID: PMC3561912
- DOI: 10.1093/hmg/dds496
MicroRNA-21 silencing enhances the cytotoxic effect of the antiangiogenic drug sunitinib in glioblastoma
Abstract
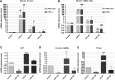
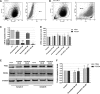
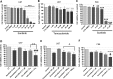
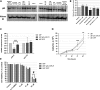
Highly malignant glioblastoma (GBM) is characterized by high genetic heterogeneity and infiltrative brain invasion patterns, and aberrant miRNA expression has been associated with hallmark malignant properties of GBM. The lack of effective GBM treatment options prompted us to investigate whether miRNAs would constitute promising therapeutic targets toward the generation of a gene therapy approach with clinical significance for this disease. Here, we show that microRNA-21 (miR-21) is upregulated and microRNA-128 (miR-128) is downregulated in mouse and human GBM samples, a finding that is corroborated by analysis of a large set of human GBM data from The Cancer Genome Atlas. Moreover, we demonstrate that oligonucleotide-mediated miR-21 silencing in U87 human GBM cells resulted in increased levels of the tumor suppressors PTEN and PDCD4, caspase 3/7 activation and decreased tumor cell proliferation. Cell exposure to pifithrin, an inhibitor of p53 transcriptional activity, reduced the caspase activity associated with decreased miR-21 expression. Finally, we demonstrate for the first time that miR-21 silencing enhances the antitumoral effect of the tyrosine kinase inhibitor sunitinib, whereas no therapeutic benefit is observed when coupling miR-21 silencing with the first-line drug temozolomide. Overall, our results provide evidence that miR-21 is uniformly overexpressed in GBM and constitutes a highly promising target for multimodal therapeutic approaches toward GBM.
Figures



References
-
- Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. - PubMed
-
- Khasraw M., Lassman A.B. Advances in the treatment of malignant gliomas. Curr. Oncol. Rep. 2010;12:26–33. - PubMed
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Bartel D.P., Chen C.Z. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat. Rev. Genet. 2004;5:396–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

