Corneal afferents differentially target thalamic- and parabrachial-projecting neurons in spinal trigeminal nucleus caudalis
- PMID: 23201828
- PMCID: PMC3620795
- DOI: 10.1016/j.neuroscience.2012.11.033
Corneal afferents differentially target thalamic- and parabrachial-projecting neurons in spinal trigeminal nucleus caudalis
Abstract
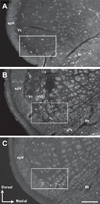
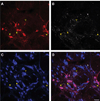
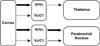
Dorsal horn neurons send ascending projections to both thalamic nuclei and parabrachial nuclei; these pathways are thought to be critical pathways for central processing of nociceptive information. Afferents from the corneal surface of the eye mediate nociception from this tissue which is susceptible to clinically important pain syndromes. This study examined corneal afferents to the trigeminal dorsal horn and compared inputs to thalamic- and parabrachial-projecting neurons. We used anterograde tracing with cholera toxin B subunit to identify corneal afferent projections to trigeminal dorsal horn, and the retrograde tracer FluoroGold to identify projection neurons. Studies were conducted in adult male Sprague-Dawley rats. Our analysis was conducted at two distinct levels of the trigeminal nucleus caudalis (Vc) which receive corneal afferent projections. We found that corneal afferents project more densely to the rostral pole of Vc than the caudal pole. We also quantified the number of thalamic- and parabrachial-projecting neurons in the regions of Vc that receive corneal afferents. Corneal afferent inputs to both groups of projection neurons were also more abundant in the rostral pole of Vc. Finally, by comparing the frequency of corneal afferent appositions to thalamic- versus parabrachial-projecting neurons, we found that corneal afferents preferentially target parabrachial-projecting neurons in trigeminal dorsal horn. These results suggest that nociceptive pain from the cornea may be primarily mediated by a non-thalamic ascending pathway.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no known conflicts of interest.
Figures





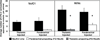
References
-
- Aicher SA, Mitchell JL, Swanson KC, Zadina JE. Endomorphin-2 axon terminals contact mu-opioid receptor-containing dendrites in trigeminal dorsal horn. Brain Res. 2003;977:190–198. - PubMed
-
- Aicher SA, Reis DJ, Nicolae R, Milner TA. Monosynaptic projections from the medullary gigantocellular reticular formation to sympathetic preganglionic neurons in the thoracic spinal cord. J Comp Neurol. 1995;363:563–580. - PubMed
-
- Allen GV, Barbrick B, Esser MJ. Trigeminal-parabrachial connections: Possible pathway for nociception-induced cardiovascular reflex responses. Brain Res. 1996;715:125–135. - PubMed
-
- Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

