The swipe card model of odorant recognition
- PMID: 23202229
- PMCID: PMC3522982
- DOI: 10.3390/s121115709
The swipe card model of odorant recognition
Abstract
Just how we discriminate between the different odours we encounter is not completely understood yet. While obviously a matter involving biology, the core issue isa matter for physics: what microscopic interactions enable the receptors in our noses-small protein switches—to distinguish scent molecules? We survey what is and is not known about the physical processes that take place when we smell things, highlighting the difficulties in developing a full understanding of the mechanics of odorant recognition. The main current theories, discussed here, fall into two major groups. One class emphasises the scent molecule's shape, and is described informally as a "lock and key" mechanism. But there is another category, which we focus on and which we call "swipe card" theories:the molecular shape must be good enough, but the information that identifies the smell involves other factors. One clearly-defined "swipe card" mechanism that we discuss here is Turin's theory, in which inelastic electron tunnelling is used to discern olfactant vibration frequencies. This theory is explicitly quantal, since it requires the molecular vibrations to take in or give out energy only in discrete quanta. These ideas lead to obvious experimental tests and challenges. We describe the current theory in a form that takes into account molecular shape as well as olfactant vibrations. It emerges that this theory can explain many observations hard to reconcile in other ways. There are still some important gaps in a comprehensive physics-based description of the central steps in odorant recognition. We also discuss how far these ideas carry over to analogous processes involving other small biomolecules, like hormones, steroids and neurotransmitters. We conclude with a discussion of possible quantum behaviours in biology more generally, the case of olfaction being just one example. This paper is presented in honour of Prof. Marshall Stoneham who passed away unexpectedly during its writing.
Figures
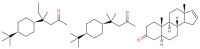







References
-
- Lewis A., Del Prioire L.V. The biophysics of visual photoreception. Phys. Today. 1988;41:38–46.
-
- Malnic B., Hirono J., Sato T., Buck L.B. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. - PubMed
-
- Buck L., Axel R. A novel multi-gene family may encode odorant receptors. Cell. 1991;65:175–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

