Pharmacophore selection and redesign of non-nucleotide inhibitors of anthrax edema factor
- PMID: 23202316
- PMCID: PMC3509708
- DOI: 10.3390/toxins4111288
Pharmacophore selection and redesign of non-nucleotide inhibitors of anthrax edema factor
Abstract
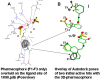
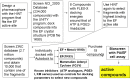
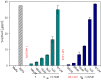
Antibiotic treatment may fail to protect individuals, if not started early enough, after infection with Bacillus anthracis, due to the continuing activity of toxins that the bacterium produces. Stable and easily stored inhibitors of the edema factor toxin (EF), an adenylyl cyclase, could save lives in the event of an outbreak, due to natural causes or a bioweapon attack. The toxin's basic activity is to convert ATP to cAMP, and it is thus in principle a simple phosphatase, which means that many mammalian enzymes, including intracellular adenylcyclases, may have a similar activity. While nucleotide based inhibitors, similar to its natural substrate, ATP, were identified early, these compounds had low activity and specificity for EF. We used a combined structural and computational approach to choose small organic molecules in large, web-based compound libraries that would, based on docking scores, bind to residues within the substrate binding pocket of EF. A family of fluorenone-based inhibitors was identified that inhibited the release of cAMP from cells treated with EF. The lead inhibitor was also shown to inhibit the diarrhea caused by enterotoxigenic E. coli (ETEC) in a murine model, perhaps by serving as a quorum sensor. These inhibitors are now being tested for their ability to inhibit Anthrax infection in animal models and may have use against other pathogens that produce toxins similar to EF, such as Bordetella pertussis or Vibrio cholera.
Figures

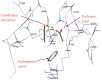
References
-
- Migone T.S., Subramanian G.M., Zhong J., Healey L.M., Corey A., Devalaraja M., Lo L., Ullrich S., Zimmerman J., Chen A., et al. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 2009;361:135–144. - PubMed
-
- Sweeney D.A., Cui X., Solomon S.B., Vitberg D.A., Migone T.S., Scher D., Danner R.L., Natanson C., Subramanian G.M., Eichacker P.Q. Anthrax lethal and edema toxins produce different patterns of cardiovascular and renal dysfunction and synergistically decrease survival in canines. J. Infect. Dis. 2010;202:1885–1896. doi: 10.1086/657408. - DOI - PMC - PubMed
-
- Lacy D.B., Collier R.J. Structure and function of anthrax toxin. Curr. Top. Microbiol. Immunol. 2002;271:61–85. - PubMed
-
- Lacy D.B., Mourez M., Fouassier A., Collier R.J. Mapping the anthrax protective antigen binding site on the lethal and edema factors. J. Biol. Chem. 2002;277:3006–3010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

