Zwitterionic polymer-modified silicon microring resonators for label-free biosensing in undiluted human plasma
- PMID: 23202337
- PMCID: PMC3832353
- DOI: 10.1016/j.bios.2012.10.079
Zwitterionic polymer-modified silicon microring resonators for label-free biosensing in undiluted human plasma
Abstract
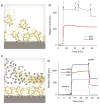
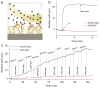
A widely acknowledged goal in personalized medicine is to radically reduce the costs of highly parallelized, small fluid volume, point-of-care and home-based diagnostics. Recently, there has been a surge of interest in using complementary metal-oxide-semiconductor (CMOS)-compatible silicon photonic circuits for biosensing, with the promise of producing chip-scale integrated devices containing thousands of orthogonal sensors, at minimal cost on a per-chip basis. A central challenge in biosensor translation is to engineer devices that are both sensitive and specific to a target analyte within unprocessed biological fluids. Despite advances in the sensitivity of silicon photonic biosensors, poor biological specificity at the sensor surface remains a significant factor limiting assay performance in complex media (i.e. whole blood, plasma, serum) due to the non-specific adsorption of proteins and other biomolecules. Here, we chemically modify the surface of silicon microring resonator biosensors for the label-free detection of an analyte in undiluted human plasma. This work highlights the first application of a non-fouling zwitterionic surface coating to enable silicon photonic-based label-free detection of a protein analyte at clinically relevant sensitivities in undiluted human plasma.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Label-free quantitation of a cancer biomarker in complex media using silicon photonic microring resonators.Anal Chem. 2009 Nov 15;81(22):9499-506. doi: 10.1021/ac902006p. Anal Chem. 2009. PMID: 19848413 Free PMC article.
-
Label-free electrical detection of cardiac biomarker with complementary metal-oxide semiconductor-compatible silicon nanowire sensor arrays.Anal Chem. 2009 Aug 1;81(15):6266-71. doi: 10.1021/ac901157x. Anal Chem. 2009. PMID: 20337397
-
Silicon Photonic Biosensors Using Label-Free Detection.Sensors (Basel). 2018 Oct 18;18(10):3519. doi: 10.3390/s18103519. Sensors (Basel). 2018. PMID: 30340405 Free PMC article. Review.
-
Sensitive on-chip detection of a protein biomarker in human serum and plasma over an extended dynamic range using silicon photonic microring resonators and sub-micron beads.Lab Chip. 2011 Jun 21;11(12):2042-4. doi: 10.1039/c1lc20231f. Epub 2011 May 4. Lab Chip. 2011. PMID: 21541438 Free PMC article.
-
Biofunctionalization of Multiplexed Silicon Photonic Biosensors.Biosensors (Basel). 2022 Dec 29;13(1):53. doi: 10.3390/bios13010053. Biosensors (Basel). 2022. PMID: 36671887 Free PMC article. Review.
Cited by
-
Direct measurement and analytical description of the mode alignment in inversely tapered silicon nano-resonators.Sci Rep. 2019 Jun 21;9(1):9024. doi: 10.1038/s41598-019-45034-0. Sci Rep. 2019. PMID: 31227720 Free PMC article.
-
Next generation ligand binding assays-review of emerging real-time measurement technologies.AAPS J. 2014 Sep;16(5):914-24. doi: 10.1208/s12248-014-9643-2. Epub 2014 Jul 25. AAPS J. 2014. PMID: 25060773 Free PMC article. Review.
-
Biomolecular analysis with microring resonators: applications in multiplexed diagnostics and interaction screening.Curr Opin Chem Biol. 2013 Oct;17(5):818-26. doi: 10.1016/j.cbpa.2013.06.014. Epub 2013 Jul 17. Curr Opin Chem Biol. 2013. PMID: 23871688 Free PMC article. Review.
-
Integrating Nanostructured Artificial Receptors with Whispering Gallery Mode Optical Microresonators via Inorganic Molecular Imprinting Techniques.Biosensors (Basel). 2016 Jun 15;6(2):26. doi: 10.3390/bios6020026. Biosensors (Basel). 2016. PMID: 27314397 Free PMC article.
-
Using glycan microarrays to understand immunity.Curr Opin Chem Biol. 2014 Feb;18:55-61. doi: 10.1016/j.cbpa.2013.12.017. Epub 2014 Jan 29. Curr Opin Chem Biol. 2014. PMID: 24486647 Free PMC article. Review.
References
-
- Armani AM, Kulkarni RP, Fraser SE, Flagan RC, Vahala KJ. Science. 2007;317 (5839):783. - PubMed
-
- Arnold S, Khoshsima M, Teraoka I, Holler S, Vollmer F. Optics Letters. 2003;28 (4):272–274. - PubMed
-
- Brault ND, Gao C, Xue H, Piliarik M, Homola J, Jiang S, Yu Q. Biosensors and Bioelectronics. 2010;25 (10):2276–2282. - PubMed
-
- Chen S, Zheng J, Li L, Jiang S. Journal of the American Chemical Society. 2005;127 (41):14473–14478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

