Conversion of human fibroblasts to angioblast-like progenitor cells
- PMID: 23202434
- PMCID: PMC3531579
- DOI: 10.1038/nmeth.2255
Conversion of human fibroblasts to angioblast-like progenitor cells
Erratum in
-
Author Correction: Conversion of human fibroblasts to angioblast-like progenitor cells.Nat Methods. 2020 Mar;17(3):353. doi: 10.1038/s41592-020-0745-8. Nat Methods. 2020. PMID: 32034376
Abstract
Lineage conversion of one somatic cell type to another is an attractive approach for generating specific human cell types. Lineage conversion can be direct, in the absence of proliferation and multipotent progenitor generation, or indirect, by the generation of expandable multipotent progenitor states. We report the development of a reprogramming methodology in which cells transition through a plastic intermediate state, induced by brief exposure to reprogramming factors, followed by differentiation. We use this approach to convert human fibroblasts to mesodermal progenitor cells, including by non-integrative approaches. These progenitor cells demonstrated bipotent differentiation potential and could generate endothelial and smooth muscle lineages. Differentiated endothelial cells exhibited neo-angiogenesis and anastomosis in vivo. This methodology for indirect lineage conversion to angioblast-like cells adds to the armamentarium of reprogramming approaches aimed at the study and treatment of ischemic pathologies.
Figures

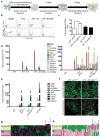
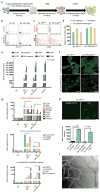

Comment in
-
An indirect approach to generating specific human cell types.Nat Methods. 2013 Jan;10(1):44-5. doi: 10.1038/nmeth.2325. Nat Methods. 2013. PMID: 23269377 Free PMC article.
References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–744. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

