Advanced vaccine candidates for Lassa fever
- PMID: 23202493
- PMCID: PMC3509661
- DOI: 10.3390/v4112514
Advanced vaccine candidates for Lassa fever
Abstract
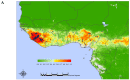
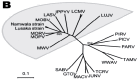
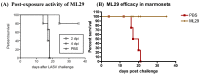
Lassa virus (LASV) is the most prominent human pathogen of the Arenaviridae. The virus is transmitted to humans by a rodent reservoir, Mastomys natalensis, and is capable of causing lethal Lassa Fever (LF). LASV has the highest human impact of any of the viral hemorrhagic fevers (with the exception of Dengue Fever) with an estimated several hundred thousand infections annually, resulting in thousands of deaths in Western Africa. The sizeable disease burden, numerous imported cases of LF in non-endemic countries, and the possibility that LASV can be used as an agent of biological warfare make a strong case for vaccine development. Presently there is no licensed vaccine against LF or approved treatment. Recently, several promising vaccine candidates have been developed which can potentially target different groups at risk. The purpose of this manuscript is to review the LASV pathogenesis and immune mechanisms involved in protection. The current status of pre-clinical development of the advanced vaccine candidates that have been tested in non-human primates will be discussed. Major scientific, manufacturing, and regulatory challenges will also be considered.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

