Mirror-symmetric microtubule assembly and cell interactions drive lumen formation in the zebrafish neural rod
- PMID: 23202854
- PMCID: PMC3545300
- DOI: 10.1038/emboj.2012.305
Mirror-symmetric microtubule assembly and cell interactions drive lumen formation in the zebrafish neural rod
Abstract
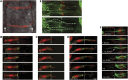
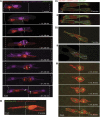
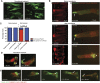
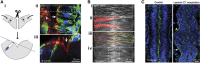
By analysing the cellular and subcellular events that occur in the centre of the developing zebrafish neural rod, we have uncovered a novel mechanism of cell polarisation during lumen formation. Cells from each side of the neural rod interdigitate across the tissue midline. This is necessary for localisation of apical junctional proteins to the region where cells intersect the tissue midline. Cells assemble a mirror-symmetric microtubule cytoskeleton around the tissue midline, which is necessary for the trafficking of proteins required for normal lumen formation, such as partitioning defective 3 and Rab11a to this point. This occurs in advance and is independent of the midline cell division that has been shown to have a powerful role in lumen organisation. To our knowledge, this is the first example of the initiation of apical polarisation part way along the length of a cell, rather than at a cell extremity. Although the midline division is not necessary for apical polarisation, it confers a morphogenetic advantage by efficiently eliminating cellular processes that would otherwise bridge the developing lumen.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



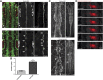
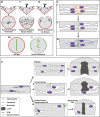
Comment in
-
Neurulation: coordinating cell polarisation and lumen formation.EMBO J. 2013 Jan 9;32(1):1-3. doi: 10.1038/emboj.2012.325. Epub 2012 Dec 4. EMBO J. 2013. PMID: 23211745 Free PMC article.
References
-
- Baena-Lopez LA, Baonza A, Garcia-Bellido A (2005) The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol 15: 1640–1644 - PubMed
-
- Bellett G, Carter JM, Keynton J, Goldspink D, James C, Moss DK, Mogensen MM (2009) Microtubule plus-end and minus-end capture at adherens junctions is involved in the assembly of apico-basal arrays in polarised epithelial cells. Cell Motil Cytoskeleton 66: 893–908 - PubMed
-
- Cai Y, Yu F, Lin S, Chia W, Yang X (2003) Apical complex genes control mitotic spindle geometry and relative size of daughter cells in Drosophila neuroblast and pI asymmetric divisions. Cell 112: 51–62 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

