Theoretical models for surface forces and adhesion and their measurement using atomic force microscopy
- PMID: 23202925
- PMCID: PMC3497299
- DOI: 10.3390/ijms131012773
Theoretical models for surface forces and adhesion and their measurement using atomic force microscopy
Abstract
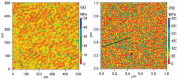
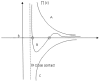
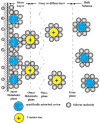
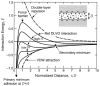
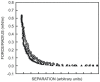
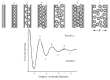
The increasing importance of studies on soft matter and their impact on new technologies, including those associated with nanotechnology, has brought intermolecular and surface forces to the forefront of physics and materials science, for these are the prevailing forces in micro and nanosystems. With experimental methods such as the atomic force spectroscopy (AFS), it is now possible to measure these forces accurately, in addition to providing information on local material properties such as elasticity, hardness and adhesion. This review provides the theoretical and experimental background of afs, adhesion forces, intermolecular interactions and surface forces in air, vacuum and in solution.
Figures

















References
-
- Burnham N.A., Kulik A.J. Surface Forces and Adhesion. In: Bhushan B., editor. Handbook of Micro/Nanotribology. CRC Press, LLC; Boca Raton, FL, USA: 1999.
-
- Myers D. Surfaces, Interfaces, and Colloids: Principles and Applications. 2nd ed. Wiley-VCH; New York, NY, USA: 1999. p. 501.
-
- Varandas A.J.C., Brandao J. A simple semi-empirical approach to the intermolecular potential of vanderwaals systems.1. Isotropic interactions—Application to the lowest triplet-state of the alkali dimers. Mol. Phys. 1982;45:857–875.
-
- Van der Waals J.D. Thermodynamische Theorie der Capillariteit in de Onderstelling van Continue Dichtheidsverandering. Vol. 1. Verhandel Konink Akad Weten; Amsterdam, The Netherlands: 1893. p. 56.
-
- Kitchener J.A., Prosser A.P. Direct measurement of the long-range van der waals forces. Proc. R. Soc. A. 1957;242:403–409.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

