Probing nucleic acid interactions and pre-mRNA splicing by Förster Resonance Energy Transfer (FRET) microscopy
- PMID: 23203103
- PMCID: PMC3509619
- DOI: 10.3390/ijms131114929
Probing nucleic acid interactions and pre-mRNA splicing by Förster Resonance Energy Transfer (FRET) microscopy
Abstract
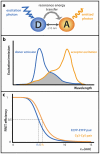
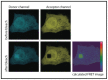
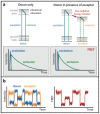
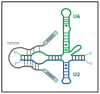
Förster resonance energy transfer (FRET) microscopy is a powerful technique routinely used to monitor interactions between biomolecules. Here, we focus on the techniques that are used for investigating the structure and interactions of nucleic acids (NAs). We present a brief overview of the most commonly used FRET microscopy techniques, their advantages and drawbacks. We list experimental approaches recently used for either in vitro or in vivo studies. Next, we summarize how FRET contributed to the understanding of pre-mRNA splicing and spliceosome assembly.
Figures




Similar articles
-
Methodologies for studying the spliceosome's RNA dynamics with single-molecule FRET.Methods. 2017 Aug 1;125:45-54. doi: 10.1016/j.ymeth.2017.05.011. Epub 2017 May 18. Methods. 2017. PMID: 28529063 Free PMC article. Review.
-
Single-Molecule Pull-Down FRET to Dissect the Mechanisms of Biomolecular Machines.Methods Enzymol. 2015;558:539-570. doi: 10.1016/bs.mie.2015.01.009. Epub 2015 Mar 3. Methods Enzymol. 2015. PMID: 26068753 Free PMC article.
-
Molecular choreography of pre-mRNA splicing by the spliceosome.Curr Opin Struct Biol. 2019 Dec;59:124-133. doi: 10.1016/j.sbi.2019.07.010. Epub 2019 Aug 30. Curr Opin Struct Biol. 2019. PMID: 31476650 Review.
-
Conformational dynamics of stem II of the U2 snRNA.RNA. 2016 Feb;22(2):225-36. doi: 10.1261/rna.052233.115. Epub 2015 Dec 2. RNA. 2016. PMID: 26631165 Free PMC article.
-
Mechanistic insights into precursor messenger RNA splicing by the spliceosome.Nat Rev Mol Cell Biol. 2017 Nov;18(11):655-670. doi: 10.1038/nrm.2017.86. Epub 2017 Sep 27. Nat Rev Mol Cell Biol. 2017. PMID: 28951565 Review.
Cited by
-
Bioaffinity Nanoprobes for Foodborne Pathogen Sensing.Micromachines (Basel). 2023 May 26;14(6):1122. doi: 10.3390/mi14061122. Micromachines (Basel). 2023. PMID: 37374709 Free PMC article.
-
Selective Detection of an Infection Biomarker by an Osteo-Friend Scaffold: Development of a Multifunctional Artificial Bone Substitute.Biosensors (Basel). 2021 Nov 24;11(12):473. doi: 10.3390/bios11120473. Biosensors (Basel). 2021. PMID: 34940230 Free PMC article.
-
A Critical Review on the Sensing, Control, and Manipulation of Single Molecules on Optofluidic Devices.Micromachines (Basel). 2022 Jun 18;13(6):968. doi: 10.3390/mi13060968. Micromachines (Basel). 2022. PMID: 35744582 Free PMC article. Review.
References
-
- Förster T. Energiewanderung und Fluoreszenz. Naturwissenschaften. 1946;33:166–175.
-
- Sapsford K.E., Berti L., Medintz I.L. Materials for fluorescence resonance energy transfer analysis: Beyond traditional donor-acceptor combinations. Angew. Chem. Int. Ed. Engl. 2006;45:4562–4589. - PubMed
-
- Gonçalves M.S.T. Fluorescent labeling of biomolecules with organic probes. Chem. Rev. 2009;109:190–212. - PubMed
-
- Jares-Erijman E.A., Jovin T.M. FRET imaging. Nat. Biotechnol. 2003;21:1387–1395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

