Detection of glycomic alterations induced by overexpression of p-glycoprotein on the surfaces of L1210 cells using sialic acid binding lectins
- PMID: 23203118
- PMCID: PMC3509634
- DOI: 10.3390/ijms131115177
Detection of glycomic alterations induced by overexpression of p-glycoprotein on the surfaces of L1210 cells using sialic acid binding lectins
Abstract
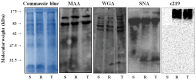
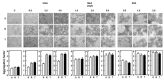
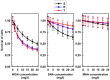
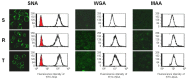
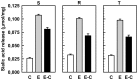
P-glycoprotein (P-gp) overexpression is the most frequently observed cause of multidrug resistance in neoplastic cells. In our experiments, P-gp was expressed in L1210 mice leukemia cells (S cells) by selection with vincristine (R cells) or transfection with the gene encoding human P-gp (T cells). Remodeling of cell surface sugars is associated with P-gp expression in L1210 cells as a secondary cellular response. In this study, we monitored the alteration of cell surface saccharides by Sambucus nigra agglutinin (SNA), wheat germ agglutinin (WGA) and Maackia amurensis agglutinin (MAA). Sialic acid is predominantly linked to the surface of S, R and T cells via α-2,6 branched sugars that tightly bind SNA. The presence of sialic acid linked to the cell surface via α-2,3 branched sugars was negligible, and the binding of MAA (recognizing this branch) was much less pronounced than SNA. WGA induced greater cell death than SNA, which was bound to the cell surface and agglutinated all three L1210 cell-variants more effectively than WGA. Thus, the ability of lectins to induce cell death did not correlate with their binding efficiency and agglutination potency. Compared to S cells, P-gp positive R and T cells contain a higher amount of N-acetyl-glucosamine on their cell surface, which is associated with improved WGA binding. Both P-gp positive variants of L1210 cells are strongly resistant to vincristine as P-gp prototypical drug. This resistance could not be altered by liberalization of terminal sialyl residues from the cell surface by sialidase.
Figures
Similar articles
-
Overexpression of GRP78/BiP in P-Glycoprotein-Positive L1210 Cells is Responsible for Altered Response of Cells to Tunicamycin as a Stressor of the Endoplasmic Reticulum.Cells. 2020 Apr 6;9(4):890. doi: 10.3390/cells9040890. Cells. 2020. PMID: 32268491 Free PMC article.
-
Vincristine-induced overexpression of P-glycoprotein in L1210 cells is associated with remodeling of cell surface saccharides.J Proteome Res. 2009 Feb;8(2):513-20. doi: 10.1021/pr8007094. J Proteome Res. 2009. PMID: 19099507
-
Multidrug resistant P-glycoprotein positive L1210/VCR cells are also cross-resistant to cisplatin via a mechanism distinct from P-glycoprotein-mediated drug efflux activity.Gen Physiol Biophys. 2009 Dec;28(4):391-403. doi: 10.4149/pb_2009_04_391. Gen Physiol Biophys. 2009. PMID: 20097962
-
Effect of thapsigargin on P-glycoprotein-negative and P-glycoprotein-positive L1210 mouse leukaemia cells.Gen Physiol Biophys. 2010 Dec;29(4):396-401. doi: 10.4149/gpb_2010_04_396. Gen Physiol Biophys. 2010. PMID: 21157003
-
Human zona pellucida recognition associated with removal of sialic acid from human sperm surface.J Reprod Fertil. 1994 Aug;101(3):703-11. doi: 10.1530/jrf.0.1010703. J Reprod Fertil. 1994. PMID: 7966029
Cited by
-
Establishment and Characteristics of a Novel Mantle Cell Lymphoma-derived Cell Line and a Bendamustine-resistant Subline.Cancer Genomics Proteomics. 2018 May-Jun;15(3):213-223. doi: 10.21873/cgp.20080. Cancer Genomics Proteomics. 2018. PMID: 29695404 Free PMC article.
-
Do wolframin, P-glycoprotein, and GRP78/BiP cooperate to alter the response of L1210 cells to endoplasmic reticulum stress or drug sensitivity?Cancer Cell Int. 2025 Feb 7;25(1):35. doi: 10.1186/s12935-025-03661-w. Cancer Cell Int. 2025. PMID: 39920654 Free PMC article.
-
In-situ profiling of glycosylation on single cells with surface plasmon resonance imaging.Nat Commun. 2025 Jan 24;16(1):1000. doi: 10.1038/s41467-025-56390-z. Nat Commun. 2025. PMID: 39856037 Free PMC article.
-
Overexpression of GRP78/BiP in P-Glycoprotein-Positive L1210 Cells is Responsible for Altered Response of Cells to Tunicamycin as a Stressor of the Endoplasmic Reticulum.Cells. 2020 Apr 6;9(4):890. doi: 10.3390/cells9040890. Cells. 2020. PMID: 32268491 Free PMC article.
-
Effects of Sulforaphane-Induced Cell Death upon Repeated Passage of Either P-Glycoprotein-Negative or P-Glycoprotein-Positive L1210 Cell Variants.Int J Mol Sci. 2022 Sep 16;23(18):10818. doi: 10.3390/ijms231810818. Int J Mol Sci. 2022. PMID: 36142752 Free PMC article.
References
-
- Perez-Tomas R. Multidrug resistance: Retrospect and prospects in anti-cancer drug treatment. Curr. Med. Chem. 2006;13:1859–1876. - PubMed
-
- Breier A., Barancik M., Sulova Z., Uhrik B. P-glycoprotein--implications of metabolism of neoplastic cells and cancer therapy. Curr. Cancer Drug Targets. 2005;5:457–468. - PubMed
-
- Breier A., Gibalova L., Seres M., Barancik M., Sulova Z. New Insight into P-Glycoprotein as a Drug Target. Anticancer Agents Med. Chem. 2012 in press. - PubMed
-
- Sulova Z., Brtko J., Macejova D., Breier A. Are Nuclear Receptors for Retinoids Involved in the Control of the Expression and Activity of P-Glycoprotein? In: Cheng L.-H., Ito Y., editors. Retinoic Acid: Structure, Mechanisms and Roles in Disease. NOVA Publisher; New York, NY, USA: 2012. pp. 29–52.
-
- Cerveny L., Svecova L., Anzenbacherova E., Vrzal R., Staud F., Dvorak Z., Ulrichova J., Anzenbacher P., Pavek P. Valproic acid induces CYP3A4 and MDR1 gene expression by activation of constitutive androstane receptor and pregnane X receptor pathways. Drug Metab. Dispos. 2007;35:1032–1041. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

