Errors in the calculation of (27)Al nuclear magnetic resonance chemical shifts
- PMID: 23203134
- PMCID: PMC3509650
- DOI: 10.3390/ijms131115420
Errors in the calculation of (27)Al nuclear magnetic resonance chemical shifts
Abstract
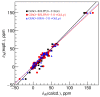
Computational chemistry is an important tool for signal assignment of 27Al nuclear magnetic resonance spectra in order to elucidate the species of aluminum(III) in aqueous solutions. The accuracy of the popular theoretical models for computing the 27Al chemical shifts was evaluated by comparing the calculated and experimental chemical shifts in more than one hundred aluminum(III) complexes. In order to differentiate the error due to the chemical shielding tensor calculation from that due to the inadequacy of the molecular geometry prediction, single-crystal X-ray diffraction determined structures were used to build the isolated molecule models for calculating the chemical shifts. The results were compared with those obtained using the calculated geometries at the B3LYP/6-31G(d) level. The isotropic chemical shielding constants computed at different levels have strong linear correlations even though the absolute values differ in tens of ppm. The root-mean-square difference between the experimental chemical shifts and the calculated values is approximately 5 ppm for the calculations based on the X-ray structures, but more than 10 ppm for the calculations based on the computed geometries. The result indicates that the popular theoretical models are adequate in calculating the chemical shifts while an accurate molecular geometry is more critical.
Figures




Similar articles
-
Aluminium siting in the ZSM-5 framework by combination of high resolution 27Al NMR and DFT/MM calculations.Phys Chem Chem Phys. 2009 Feb 28;11(8):1237-47. doi: 10.1039/b807755j. Epub 2008 Dec 12. Phys Chem Chem Phys. 2009. PMID: 19209368
-
Nuclear magnetic resonance shielding constants and chemical shifts in linear 199Hg compounds: a comparison of three relativistic computational methods.J Chem Phys. 2011 Jul 28;135(4):044306. doi: 10.1063/1.3608153. J Chem Phys. 2011. PMID: 21806118
-
Computational studies of 13C NMR chemical shifts of saccharides.Phys Chem Chem Phys. 2005 Jul 7;7(13):2561-9. doi: 10.1039/b505546f. Epub 2005 Jun 9. Phys Chem Chem Phys. 2005. PMID: 16189565
-
Aluminum binding to phosphatidylcholine lipid bilayer membranes: 27Al and 31P NMR spectroscopic studies.Chem Phys Lipids. 2004 Nov;132(1):23-36. doi: 10.1016/j.chemphyslip.2004.09.003. Chem Phys Lipids. 2004. PMID: 15530445
-
Assessment of the accuracy of theoretical methods for calculating (27)Al nuclear magnetic resonance shielding tensors of aquated aluminum species.J Phys Chem A. 2009 Apr 30;113(17):5138-43. doi: 10.1021/jp810632f. J Phys Chem A. 2009. PMID: 19344113
Cited by
-
Biomass-mediated ZSM-5 zeolite synthesis: when self-assembly allows to cross the Si/Al lower limit.Chem Sci. 2018 Jul 5;9(31):6532-6539. doi: 10.1039/c8sc01675e. eCollection 2018 Aug 21. Chem Sci. 2018. PMID: 30310584 Free PMC article.
References
-
- Lewis T. Environmental Chemistry and Toxicology of Aluminium. 1st ed. CRC Press; Boca Raton, FL, USA: 1989.
-
- Sposito G. The Environmental Chemistry of Aluminum. 2nd ed. CRC Press; Boca Raton, FL, USA: 1995.
-
- Wang X.L., Zou G., Bi S. Advances in determination of aluminum in environmental and biological materials by 27Al nuclear magnetic resonance spectroscopy. Chin. J. Inorg. Chem. 2000;16:548–560.
-
- Tossell J.A. The effects of hydrolysis and oligomerization upon the NMR shieldings of Be+2 and Al+3 species in aqueous solution. J. Magn. Reson. 1998;135:203–207. - PubMed
-
- Ditchfield R. Self-consistent perturbation theory of diamagnetism. Mol. Phys. 1974;27:789–807.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

