Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer
- PMID: 23203246
- PMCID: PMC3742115
- DOI: 10.2967/jnumed.112.104661
Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer
Abstract
Prostate-specific membrane antigen (PSMA) is a type II integral membrane protein expressed on the surface of prostate cancer (PCa) cells, particularly in androgen-independent, advanced, and metastatic disease. Previously, we demonstrated that N-[N-[(S)-1,3-dicarboxypropyl]carbamoyl]-4-(18)F-fluorobenzyl-L-cysteine ((18)F-DCFBC) could image an experimental model of PSMA-positive PCa using PET. Here, we describe the initial clinical experience and radiation dosimetry of (18)F-DCFBC in men with metastatic PCa.
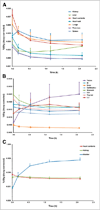
Methods: Five patients with radiologic evidence of metastatic PCa were studied after the intravenous administration of 370 MBq (10 mCi) of (18)F-DCFBC. Serial PET was performed until 2 h after administration. Time-activity curves were generated for selected normal tissues and metastatic foci. Radiation dose estimates were calculated using OLINDA/EXM 1.1.
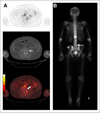
Results: Most vascular organs demonstrated a slow decrease in radioactivity concentration over time consistent with clearance from the blood pool, with primarily urinary radiotracer excretion. Thirty-two PET-positive suspected metastatic sites were identified, with 21 concordant on both PET and conventional imaging for abnormal findings compatible with metastatic disease. Of the 11 PET-positive sites not identified on conventional imaging, most were within the bone and could be considered suggestive for the detection of early bone metastases, although further validation is needed. The highest mean absorbed dose per unit administered radioactivity (μGy/MBq) was in the bladder wall (32.4), and the resultant effective dose was 19.9 ± 1.34 μSv/MBq (mean ± SD).
Conclusion: Although further studies are needed for validation, our findings demonstrate the potential of (18)F-DCFBC as a new positron-emitting imaging agent for the detection of metastatic PCa. This study also provides dose estimates for (18)F-DCFBC that are comparable to those of other PET radiopharmaceuticals such as (18)F-FDG.
Figures




References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Walczak JR, Carducci MA. Prostate cancer: a practical approach to current management of recurrent disease. Mayo Clin Proc. 2007;82:243–249. - PubMed
-
- Beltran H, Beer TM, Carducci MA, et al. New therapies for castration-resistant prostate cancer: efficacy and safety. Eur Urol. 2011;60:279–290. - PubMed
-
- Chang SS, Reuter VE, Heston WD, Gaudin PB. Metastatic renal cell carcinoma neovasculature expresses prostate-specific membrane antigen. Urology. 2001;57:801–805. - PubMed
-
- Wright GL, Jr, Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
