Regulation of fibroblast growth factor receptor signalling and trafficking by Src and Eps8
- PMID: 23203811
- PMCID: PMC3613183
- DOI: 10.1242/jcs.116228
Regulation of fibroblast growth factor receptor signalling and trafficking by Src and Eps8
Abstract
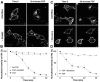
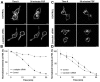
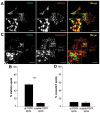
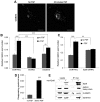
Fibroblast growth factor receptors (FGFRs) mediate a wide spectrum of cellular responses that are crucial for development and wound healing. However, aberrant FGFR activity leads to cancer. Activated growth factor receptors undergo stimulated endocytosis, but can continue to signal along the endocytic pathway. Endocytic trafficking controls the duration and intensity of signalling, and growth factor receptor signalling can lead to modifications of trafficking pathways. We have developed live-cell imaging methods for studying FGFR dynamics to investigate mechanisms that coordinate the interplay between receptor trafficking and signal transduction. Activated FGFR enters the cell following recruitment to pre-formed clathrin-coated pits (CCPs). However, FGFR activation stimulates clathrin-mediated endocytosis; FGF treatment increases the number of CCPs, including those undergoing endocytosis, and this effect is mediated by Src and its phosphorylation target Eps8. Eps8 interacts with the clathrin-mediated endocytosis machinery and depletion of Eps8 inhibits FGFR trafficking and immediate Erk signalling. Once internalized, FGFR passes through peripheral early endosomes en route to recycling and degredative compartments, through an Src- and Eps8-dependent mechanism. Thus Eps8 functions as a key coordinator in the interplay between FGFR signalling and trafficking. This work provides the first detailed mechanistic analysis of growth factor receptor clustering at the cell surface through signal transduction and endocytic trafficking. As we have characterised the Src target Eps8 as a key regulator of FGFR signalling and trafficking, and identified the early endocytic system as the site of Eps8-mediated effects, this work provides novel mechanistic insight into the reciprocal regulation of growth factor receptor signalling and trafficking.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

