Cell selective cardiovascular biology of microsomal prostaglandin E synthase-1
- PMID: 23204105
- PMCID: PMC3546279
- DOI: 10.1161/CIRCULATIONAHA.112.119479
Cell selective cardiovascular biology of microsomal prostaglandin E synthase-1
Abstract
Background: Global deletion of microsomal prostaglandin E synthase 1 (mPGES-1) in mice attenuates the response to vascular injury without a predisposition to thrombogenesis or hypertension. However, enzyme deletion results in cell-specific differential use by prostaglandin synthases of the accumulated prostaglandin H(2) substrate. Here, we generated mice deficient in mPGES-1 in vascular smooth muscle cells, endothelial cells, and myeloid cells further to elucidate the cardiovascular function of this enzyme.
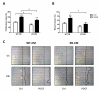
Methods and results: Vascular smooth muscle cell and endothelial cell mPGES-1 deletion did not alter blood pressure at baseline or in response to a high-salt diet. The propensity to evoked macrovascular and microvascular thrombogenesis was also unaltered. However, both vascular smooth muscle cell and endothelial cell mPGES-1-deficient mice exhibited a markedly exaggerated neointimal hyperplastic response to wire injury of the femoral artery in comparison to their littermate controls. The hyperplasia was associated with increased proliferating cell nuclear antigen and tenascin-C expression. In contrast, the response to injury was markedly suppressed by myeloid cell depletion of mPGES-1 with decreased hyperplasia, leukocyte infiltration, and expression of proliferating cell nuclear antigen and tenascin-C. Conditioned medium derived from mPGES-1-deficient macrophages less potently induced vascular smooth muscle cell proliferation and migration than that from wild-type macrophages.
Conclusions: Deletion of mPGES-1 in the vasculature and myeloid cells differentially modulates the response to vascular injury, implicating macrophage mPGES-1 as a cardiovascular drug target.
Figures


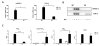






Similar articles
-
Microsomal prostaglandin e2 synthase-1 modulates the response to vascular injury.Circulation. 2011 Feb 15;123(6):631-9. doi: 10.1161/CIRCULATIONAHA.110.973685. Epub 2011 Jan 31. Circulation. 2011. PMID: 21282500 Free PMC article.
-
Myeloid cell microsomal prostaglandin E synthase-1 fosters atherogenesis in mice.Proc Natl Acad Sci U S A. 2014 May 6;111(18):6828-33. doi: 10.1073/pnas.1401797111. Epub 2014 Apr 21. Proc Natl Acad Sci U S A. 2014. PMID: 24753592 Free PMC article.
-
Protective Role of mPGES-1 (Microsomal Prostaglandin E Synthase-1)-Derived PGE2 (Prostaglandin E2) and the Endothelial EP4 (Prostaglandin E Receptor) in Vascular Responses to Injury.Arterioscler Thromb Vasc Biol. 2018 May;38(5):1115-1124. doi: 10.1161/ATVBAHA.118.310713. Epub 2018 Mar 29. Arterioscler Thromb Vasc Biol. 2018. PMID: 29599139 Free PMC article.
-
Microsomal prostaglandin E synthase-1 and blood pressure regulation.Kidney Int. 2007 Aug;72(3):274-8. doi: 10.1038/sj.ki.5002326. Epub 2007 May 9. Kidney Int. 2007. PMID: 17495855 Review.
-
Microsomal prostaglandin E synthase-1: the inducible synthase for prostaglandin E2.Arthritis Res Ther. 2005;7(3):114-7. doi: 10.1186/ar1748. Epub 2005 Apr 6. Arthritis Res Ther. 2005. PMID: 15899061 Free PMC article. Review.
Cited by
-
Deficiency of mPGES-1 exacerbates renal fibrosis and inflammation in mice with unilateral ureteral obstruction.Am J Physiol Renal Physiol. 2017 Jan 1;312(1):F121-F133. doi: 10.1152/ajprenal.00231.2016. Epub 2016 Oct 26. Am J Physiol Renal Physiol. 2017. PMID: 27784694 Free PMC article.
-
Cyclooxygenase-2-derived prostaglandin E₂ promotes injury-induced vascular neointimal hyperplasia through the E-prostanoid 3 receptor.Circ Res. 2013 Jul 5;113(2):104-14. doi: 10.1161/CIRCRESAHA.113.301033. Epub 2013 Apr 17. Circ Res. 2013. PMID: 23595951 Free PMC article.
-
Prostanoids in Cardiac and Vascular Remodeling.Arterioscler Thromb Vasc Biol. 2024 Mar;44(3):558-583. doi: 10.1161/ATVBAHA.123.320045. Epub 2024 Jan 25. Arterioscler Thromb Vasc Biol. 2024. PMID: 38269585 Free PMC article. Review.
-
TACTIC: Trans-Agency Consortium for Trauma-Induced Coagulopathy.J Thromb Haemost. 2015 Jun;13 Suppl 1(0 1):S63-71. doi: 10.1111/jth.12981. J Thromb Haemost. 2015. PMID: 26149052 Free PMC article. Review.
-
Inhibition of microsomal PGE synthase-1 reduces human vascular tone by increasing PGI2 : a safer alternative to COX-2 inhibition.Br J Pharmacol. 2017 Nov;174(22):4087-4098. doi: 10.1111/bph.13939. Epub 2017 Aug 11. Br J Pharmacol. 2017. PMID: 28675448 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

