Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009-2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients
- PMID: 23204175
- PMCID: PMC3549600
- DOI: 10.1093/infdis/jis726
Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009-2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients
Abstract
Background: The impact of neuraminidase inhibitor (NAI) treatment on clinical outcomes of public health importance during the 2009-2010 pandemic has not been firmly established.
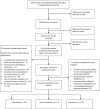
Methods: We conducted a systematic review and meta-analysis, searching 11 databases (2009 through April 2012) for relevant studies. We used standard methods conforming to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using random effects models.
Results: Regarding mortality we observed a nonsignificant reduction associated with NAI treatment (at any time) versus none (OR, 0.72 [95% CI, .51-1.01]). However we observed significant reductions for early treatment (≤48 hours after symptom onset) versus late (OR, 0.38 [95% CI, .27-.53]) and for early treatment versus none (OR, 0.35 [95% CI, .18-.71]). NAI treatment (at any time) versus none was associated with an elevated risk of severe outcome (OR, 1.76 [95% CI, 1.22-2.54]), but early versus late treatment reduced the likelihood (OR, 0.41 [95% CI, .30-.56]).
Conclusions: During the 2009-2010 influenza A(H1N1) pandemic, early initiation of NAI treatment reduced the likelihood of severe outcomes compared with late or no treatment.
Prospero registration: CRD42011001273.
Figures



Comment in
-
The beneficial effects of neuraminidase inhibitor drug therapy on severe patient outcomes during the 2009-2010 influenza A virus subtype H1N1 pandemic.J Infect Dis. 2013 Feb 15;207(4):547-9. doi: 10.1093/infdis/jis727. Epub 2012 Nov 29. J Infect Dis. 2013. PMID: 23204176 Free PMC article. No abstract available.
-
[Neuraminidase inhibitors - lessons from the swine flu].Praxis (Bern 1994). 2013 May 22;102(11):687-8. doi: 10.1024/1661-8157/a001301. Praxis (Bern 1994). 2013. PMID: 23692909 German. No abstract available.
References
-
- Makela MJ, Pauksens K, Rostila T, et al. Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J Infect. 2000;40:42–8. - PubMed
-
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group [erratum appears in Lancet 2000;356:1856] Lancet. 2000;355:1845–50. - PubMed
-
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–24. - PubMed
-
- Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children [erratum appears in Pediatr Infect Dis J 2001 20:421] Pediatr Infect Dis J. 2001;20:127–33. - PubMed
-
- Monto AS, Webster A, Keene O. Randomized, placebo-controlled studies of inhaled zanamivir in the treatment of influenza A and B: pooled efficacy analysis. J Antimicrob Chemother. 1999;44(Suppl B):23–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

