Mitogen-inducible gene 6 triggers apoptosis and exacerbates ER stress-induced β-cell death
- PMID: 23204325
- PMCID: PMC3545216
- DOI: 10.1210/me.2012-1174
Mitogen-inducible gene 6 triggers apoptosis and exacerbates ER stress-induced β-cell death
Abstract
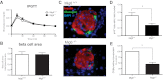
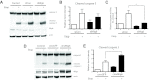
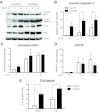
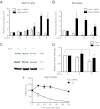
The increased insulin secretory burden placed on pancreatic β-cells during obesity and insulin resistance can ultimately lead to β-cell dysfunction and death and the development of type 2 diabetes. Mitogen-inducible gene 6 (Mig6) is a cellular stress-responsive protein that can negatively regulate the duration and intensity of epidermal growth factor receptor signaling and has been classically viewed as a molecular brake for proliferation. In this study, we used Mig6 heterozygous knockout mice (Mig6(+/-)) to study the role of Mig6 in regulating β-cell proliferation and survival. Surprisingly, the proliferation rate of Mig6(+/-) pancreatic islets was lower than wild-type islets despite having comparable β-cell mass and glucose tolerance. We thus speculated that Mig6 regulates cellular death. Using adenoviral vectors to overexpress or knockdown Mig6, we found that caspase 3 activation during apoptosis was dependent on the level of Mig6. Interestingly, Mig6 expression was induced during endoplasmic reticulum (ER) stress, and its protein levels were maintained throughout ER stress. Using polyribosomal profiling, we identified that Mig6 protein translation was maintained, whereas the global protein translation was inhibited during ER stress. In addition, Mig6 overexpression exacerbated ER stress-induced caspase 3 activation in vitro. In conclusion, Mig6 is transcriptionally up-regulated and resistant to global translational inhibition during stressed conditions in β-cells and mediates apoptosis in the form of caspase 3 activation. The sustained production of Mig6 protein exacerbates ER stress-induced β-cell death. Thus, preventing the induction, translation, and/or function of Mig6 is warranted for increasing β-cell survival.
Figures
References
-
- Yach D, Stuckler D, Brownell KD. 2006. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med 12:62–66 - PubMed
-
- Muoio DM, Newgard CB. 2008. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9:193–205 - PubMed
-
- Walter P, Ron D. 2011. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

