HDAC7 inhibits osteoclastogenesis by reversing RANKL-triggered β-catenin switch
- PMID: 23204328
- PMCID: PMC3683803
- DOI: 10.1210/me.2012-1302
HDAC7 inhibits osteoclastogenesis by reversing RANKL-triggered β-catenin switch
Abstract
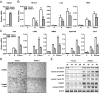
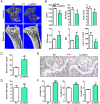
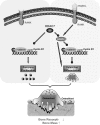
The bone-resorbing osteoclast is essential for skeletal remodeling, yet its deregulation contributes to diseases such as osteoporosis and cancer bone metastasis. Here we identify histone deacetylase 7 (HDAC7) as a key negative regulator of osteoclastogenesis and bone resorption using both in vitro cellular and molecular analyses and in vivo characterization of conditional HDAC7-knockout mice. Bone marrow osteoclast differentiation assays reveal that HDAC7 overexpression suppresses, whereas HDAC7 deletion enhances, osteoclastogenesis. Mechanistically, in the absence of receptor activator of nuclear factor κ-B ligand (RANKL), HDAC7 attenuates β-catenin function and cyclin D1 expression, thereby reducing precursor proliferation; upon RANKL activation, HDAC7 suppresses NFATc1 and prevents β-catenin down-regulation, thereby blocking osteoclast differentiation. Consequently, HDAC7 deletion in the osteoclast lineage results in a 26% reduction in bone mass (P = 0.003) owing to 102% elevated bone resorption (P = 0.01). These findings are clinically significant in light of the remarkable therapeutic potentials of HDAC inhibitors for several diseases such as cancer, diabetes, and neurodegeneration.
Figures




References
-
- Bruzzaniti A, Baron R. 2006. Molecular regulation of osteoclast activity. Rev Endocr Metab Disord 7:123–139 - PubMed
-
- Novack DV, Teitelbaum SL. 2008. The osteoclast: friend or foe? Annu Rev Pathol 3:457–484 - PubMed
-
- Wan Y. 2010. PPARγ in bone homeostasis. Trends Endocrinol Metab 21:722–728 - PubMed
-
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. 1994. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266:443–448 - PubMed
-
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. 2002. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

