Evidence for phenotypic plasticity among multihost Campylobacter jejuni and C. coli lineages, obtained using ribosomal multilocus sequence typing and Raman spectroscopy
- PMID: 23204423
- PMCID: PMC3568554
- DOI: 10.1128/AEM.02521-12
Evidence for phenotypic plasticity among multihost Campylobacter jejuni and C. coli lineages, obtained using ribosomal multilocus sequence typing and Raman spectroscopy
Abstract
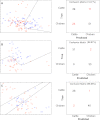
Closely related bacterial isolates can display divergent phenotypes. This can limit the usefulness of phylogenetic studies for understanding bacterial ecology and evolution. Here, we compare phenotyping based on Raman spectrometric analysis of cellular composition to phylogenetic classification by ribosomal multilocus sequence typing (rMLST) in 108 isolates of the zoonotic pathogens Campylobacter jejuni and C. coli. Automatic relevance determination (ARD) was used to identify informative peaks in the Raman spectra that could be used to distinguish strains in taxonomic and host source groups (species, clade, clonal complex, and isolate source/host). Phenotypic characterization based on Raman spectra showed a degree of agreement with genotypic classification using rMLST, with segregation accuracy between species (83.95%), clade (in C. coli, 98.41%), and, to some extent, clonal complex (86.89% C. jejuni ST-21 and ST-45 complexes) being achieved. This confirmed the utility of Raman spectroscopy for lineage classification and the correlation between genotypic and phenotypic classification. In parallel analysis, relatively distantly related isolates (different clonal complexes) were assigned the correct host origin irrespective of the clonal origin (74.07 to 96.97% accuracy) based upon different Raman peaks. This suggests that the phenotypic characteristics, from which the phenotypic signal is derived, are not fixed by clonal descent but are influenced by the host environment and change as strains move between hosts.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

