HbzF catalyzes direct hydrolysis of maleylpyruvate in the gentisate pathway of Pseudomonas alcaligenes NCIMB 9867
- PMID: 23204427
- PMCID: PMC3568541
- DOI: 10.1128/AEM.02931-12
HbzF catalyzes direct hydrolysis of maleylpyruvate in the gentisate pathway of Pseudomonas alcaligenes NCIMB 9867
Abstract
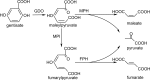
HbzF from Pseudomonas alcaligenes NCIMB 9867 was purified to homogeneity as a His-tagged protein and likely a dimer by SDS-PAGE and gel filtration. This protein was demonstrated to be a novel maleylpyruvate hydrolase, catalyzing direct hydrolysis of maleylpyruvate to maleate and pyruvate, and belongs to the fumarylacetoacetate hydrolase superfamily. This study reveals the genetic determinate for the direct maleylpyruvate hydrolysis in the gentisate pathway, complementary to the well-studied maleylpyruvate isomerization route.
Figures




References
-
- Lack L. 1959. The enzymic oxidation of gentisic acid. Biochim. Biophys. Acta 34:117–123 - PubMed
-
- Feng J, Che YS, Milse J, Yin YJ, Liu L, Ruckert C, Shen XH, Qi SW, Kalinowski J, Liu SJ. 2006. The gene ncgl2918 encodes a novel maleylpyruvate isomerase that needs mycothiol as cofactor and links mycothiol biosynthesis and gentisate assimilation in Corynebacterium glutamicum. J. Biol. Chem. 281:10778–10785 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

