Confirming stereochemical structures of strigolactones produced by rice and tobacco
- PMID: 23204500
- PMCID: PMC3548624
- DOI: 10.1093/mp/sss139
Confirming stereochemical structures of strigolactones produced by rice and tobacco
Abstract
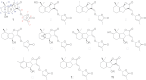
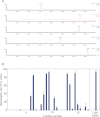
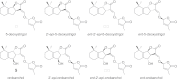
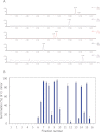
Major strigolactones (SLs) produced by rice (Oryza sativa L. cv. Nipponbare) and tobacco (Nicotiana tabacum L. cv. Michinoku No. 1) were purified and their stereochemical structures were determined by comparing with optically pure synthetic standards for their NMR and CD data and retention times and mass fragmentations in ESI-LC/MS and GC-MS. SLs purified from root exudates of rice plants were orobanchol, orobanchyl acetate, and ent-2'-epi-5-deoxystrigol. In addition to these SLs, 7-oxoorobanchyl acetate and the putative three methoxy-5-deoxystrigol isomers were detected by LC-MS/MS. The production of 7-oxoorobanchyl acetate seemed to occur in the early growth stage, as it was detected only in the root exudates collected during the first week of incubation. The root exudates of tobacco contained at least 11 SLs, including solanacol, solanacyl acetate, orobanchol, ent-2'-epi-orobanchol, orobanchyl acetate, ent-2'-epi-orobanchyl acetate, 5-deoxystrigol, ent-2'-epi-5-deoxystrigol, and three isomers of putative didehydro-orobanchol whose structures remain to be clarified. Furthermore, two sorgolactone isomers but not sorgolactone were detected as minor SLs by LC-MS/MS analysis. It is intriguing to note that rice plants produced only orobanchol-type SLs, derived from ent-2'-epi-5-deoxystrigol, but both orobanchol-type and strigol-type SLs, derived from 5-deoxystrigol were detected in tobacco plants.
Figures

References
-
- Akiyama K., Matsuzaki K., Hayashi H. (2005). Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 435, 824–827 - PubMed
-
- Bouwmeester H.J., Roux C., Lopez-Raez J.A., Bécard G. (2007). Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 12, 224–230 - PubMed
-
- Chen V.X., Boyer F.-D., Rameau C., Retailleau P., Vors J.-P., Beau J.-M. (2010). Stereochemistry, total synthesis, and biological evaluation of the new plant hormone solanacol. Chem. Eur. J. 16, 13941–13945 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

