A furoxan-amodiaquine hybrid as a potential therapeutic for three parasitic diseases()
- PMID: 23205265
- PMCID: PMC3509744
- DOI: 10.1039/C2MD20238G
A furoxan-amodiaquine hybrid as a potential therapeutic for three parasitic diseases()
Abstract
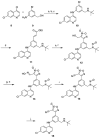
Parasitic diseases continue to have a devastating impact on human populations worldwide. Lack of effective treatments, the high cost of existing ones, and frequent emergence of resistance to these agents provide a strong argument for the development of novel therapies. Here we report the results of a hybrid approach designed to obtain a dual acting molecule that would demonstrate activity against a variety of parasitic targets. The antimalarial drug amodiaquine has been covalently joined with a nitric oxide-releasing furoxan to achieve multiple mechanisms of action. Using in vitro and ex vivo assays, the hybrid molecule shows activity against three parasites - Plasmodium falciparum, Schistosoma mansoni, and Ancylostoma ceylanicum.
Figures





References
-
- World Health Organization. Parasitic Diseases. http://www.who.int/vaccine_research/diseases/soa_parasitic/en/index.html.
-
- World Health Organization. Malaria. http://www.who.int/mediacentre/factsheets/fs094/en/
-
- World Health Organization. Schistosomiasis. http://www.who.int/mediacentre/factsheets/fs115/en/
-
- World Health Organization. Hookworm Disease. 2012 http://www.who.int/vaccine_research/diseases/soa_parasitic/en/index2.html.
-
- Andrews P, Dycka J, Frank G. Ann Trop Med Parasitol. 1980;74:167–177. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

