Delineation of breast cancer cell hierarchy identifies the subset responsible for dormancy
- PMID: 23205268
- PMCID: PMC3510468
- DOI: 10.1038/srep00906
Delineation of breast cancer cell hierarchy identifies the subset responsible for dormancy
Abstract
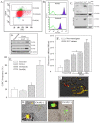
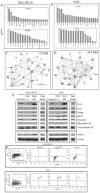
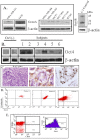
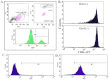
The bone marrow (BM) is a major organ of breast cancer (BC) dormancy and a common source of BC resurgence. Gap junctional intercellular communication (GJIC) between BC cells (BCCs) and BM stroma facilitates dormancy. This study reports on a hierarchy of BCCs with the most immature subset (Oct4(hi)/CD44(hi/med)/CD24(-/+)) demonstrating chemoresistance, dormancy, and stem cell properties: self-renewal, serial passaging ability, cycling quiescence, long doubling time, asymmetric division, high metastatic and invasive capability. In vitro and in vivo studies indicated that this subset was responsible for GJIC with BM stroma. Similar BCCs were detected in the blood of patients despite aggressive treatment and in a patient with a relatively large tumor but no lymph node involvement. In brief, these findings identified a novel BCC subset with stem cell properties, with preference for dormancy and in the circulation of patients. The findings establish a working cellular hierarchy of BCCs based on phenotype and functions.
Figures




Similar articles
-
Exosomes from differentially activated macrophages influence dormancy or resurgence of breast cancer cells within bone marrow stroma.Cell Death Dis. 2019 Jan 25;10(2):59. doi: 10.1038/s41419-019-1304-z. Cell Death Dis. 2019. PMID: 30683851 Free PMC article.
-
The bone marrow niche in support of breast cancer dormancy.Cancer Lett. 2016 Sep 28;380(1):263-71. doi: 10.1016/j.canlet.2015.10.033. Epub 2015 Nov 3. Cancer Lett. 2016. PMID: 26546045 Review.
-
Cell fate determination factor Dachshund reprograms breast cancer stem cell function.J Biol Chem. 2011 Jan 21;286(3):2132-42. doi: 10.1074/jbc.M110.148395. Epub 2010 Oct 11. J Biol Chem. 2011. PMID: 20937839 Free PMC article.
-
Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells.Cancer Res. 2011 Mar 1;71(5):1550-60. doi: 10.1158/0008-5472.CAN-10-2372. Epub 2011 Feb 22. Cancer Res. 2011. PMID: 21343399
-
Hierarchy of breast cancer cells: key to reverse dormancy for therapeutic intervention.Stem Cells Transl Med. 2014 Jul;3(7):782-6. doi: 10.5966/sctm.2014-0013. Epub 2014 May 15. Stem Cells Transl Med. 2014. PMID: 24833590 Free PMC article. Review.
Cited by
-
Cancer stem cell gene profile getting closer to the clinic to enhance precise treatment.EBioMedicine. 2019 Apr;42:22-23. doi: 10.1016/j.ebiom.2019.03.036. Epub 2019 Mar 20. EBioMedicine. 2019. PMID: 30904608 Free PMC article. No abstract available.
-
Evaluation of a developmental hierarchy for breast cancer cells to assess risk-based patient selection for targeted treatment.Sci Rep. 2018 Jan 10;8(1):367. doi: 10.1038/s41598-017-18834-5. Sci Rep. 2018. PMID: 29321622 Free PMC article.
-
In vitro models of cancer stem cells and clinical applications.BMC Cancer. 2016 Sep 30;16(Suppl 2):738. doi: 10.1186/s12885-016-2774-3. BMC Cancer. 2016. PMID: 27766946 Free PMC article. Review.
-
Ovarian Cancer Stem Cells: Unraveling a Germline Connection.Stem Cells Dev. 2017 Dec 15;26(24):1781-1803. doi: 10.1089/scd.2017.0153. Epub 2017 Nov 28. Stem Cells Dev. 2017. PMID: 29078734 Free PMC article.
-
Reporters of Cancer Stem Cells as a Tool for Drug Discovery.Front Oncol. 2021 Apr 22;11:669250. doi: 10.3389/fonc.2021.669250. eCollection 2021. Front Oncol. 2021. PMID: 33968778 Free PMC article. Review.
References
-
- Rao G. et al. Facilitating Role of Preprotachykinin-I Gene in the Integration of Breast Cancer Cells within the Stromal Compartment of the Bone Marrow. Cancer Res 64, 2874–2881 (2004). - PubMed
-
- King T. J., Fukushima L. H., Yasui Y., Lampe P. D. & Bertram J. S. Inducible expression of the gap junction protein connexin43 decreases the neoplastic potential of HT-1080 human fibrosarcoma cells in vitro and in vivo. Mol Carcinog 35, 29–41 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

