A soluble granulocyte colony stimulating factor decoy receptor as a novel tool to increase hematopoietic cell homing and reconstitution in mice
- PMID: 23205715
- PMCID: PMC3585478
- DOI: 10.1089/scd.2012.0438
A soluble granulocyte colony stimulating factor decoy receptor as a novel tool to increase hematopoietic cell homing and reconstitution in mice
Abstract
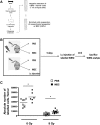
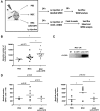
The relative ineffectiveness of hematopoietic stem cells in reaching the bone marrow upon transplantation combined with the limited number of these cells available is a major reason for graft failure and delayed hematopoietic recovery. Hence, the development of strategies that could enhance homing is of high interest. Here, we provide evidence that homing is severely impaired postexposure to ionizing radiation (IR) in mice, an effect we found was time dependent and could be partially rescued using mesenchymal stromal cell (MSC) therapy. In an attempt to further increase homing, we took advantage of our observation that the granulocyte colony stimulating factor (G-CSF), a cytokine known to induce cell mobilization, is increased in the marrow of mice shortly after their exposure to IR. As such, we developed a truncated, yet functional, soluble G-CSF receptor (solG-CSFR), which we hypothesized could act as a decoy and foster homing. Using MSCs or conditioned media as delivery vehicles, we show that an engineered solG-CSFR has the potential to increase homing and hematopoietic reconstitution in mice. Altogether, our results provide novel findings at the interplay of IR and stromal cell therapy and present the regulation of endogenous G-CSF as an innovative proof-of-concept strategy to manipulate hematopoietic cell homing.
Figures




References
-
- Rocha V. Broxmeyer HE. New approaches for improving engraftment after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:S126–S132. - PubMed
-
- Jetmore A. Plett PA. Tong X. Wolber FM. Breese R. Abonour R. Orschell-Traycoff CM. Srour EF. Homing efficiency, cell cycle kinetics, and survival of quiescent and cycling human CD34(+) cells transplanted into conditioned NOD/SCID recipients. Blood. 2002;99:1585–1593. - PubMed
-
- Petit I. Szyper-Kravitz M. Nagler A. Lahav M. Peled A. Habler L. Ponomaryov T. Taichman RS. Arenzana-Seisdedos F, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. - PubMed
-
- Sato N. Sawada K. Takahashi TA. Mogi Y. Asano S. Koike T. Sekiguchi S. A time course study for optimal harvest of peripheral blood progenitor cells by granulocyte colony-stimulating factor in healthy volunteers. Exp Hematol. 1994;22:973–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

