Profiling two indole-2-carboxamides for allosteric modulation of the CB1 receptor
- PMID: 23205875
- PMCID: PMC3578011
- DOI: 10.1111/jnc.12115
Profiling two indole-2-carboxamides for allosteric modulation of the CB1 receptor
Abstract
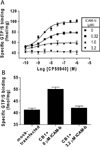
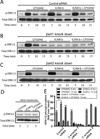
Allosteric modulation of G-protein coupled receptors (GPCRs) represents a novel approach for fine-tuning GPCR functions. The cannabinoid CB1 receptor, a GPCR associated with the CNS, has been implicated in the treatment of drug addiction, pain, and appetite disorders. We report here the synthesis and pharmacological characterization of two indole-2-carboxamides:5-chloro-3-ethyl-1-methyl-N-(4-(piperidin-1-yl)phenethyl)-1H-indole-2-carboxamide (ICAM-a) and 5-chloro-3-pentyl-N-(4-(piperidin-1-yl)phenethyl)-1H-indole-2-carboxamide (ICAM-b). Although both ICAM-a and ICAM-b enhanced CP55, 940 binding, ICAM-b exhibited the strongest positive cooperativity thus far demonstrated for enhancing agonist binding to the CB1 receptor. Although it displayed negative modulatory effects on G-protein coupling to CB1, ICAM-b induced β-arrestin-mediated downstream activation of extracellular signal-regulated kinase (ERK) signaling. These results indicate that this compound represents a novel class of CB1 ligands that produce biased signaling via CB1.
© 2012 International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

