A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression
- PMID: 23206319
- PMCID: PMC3594049
- DOI: 10.1016/j.biopsych.2012.10.019
A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression
Abstract
Background: The high-affinity N-methyl-D-aspartate (NMDA) antagonist ketamine exerts rapid antidepressant effects but has psychotomimetic properties. AZD6765 is a low-trapping NMDA channel blocker with low rates of associated psychotomimetic effects. This study investigated whether AZD6765 could produce rapid antidepressant effects in subjects with treatment-resistant major depressive disorder (MDD).
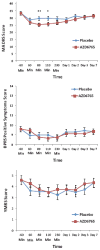
Methods: In this double-blind, randomized, crossover, placebo-controlled study, 22 subjects with DSM-IV treatment-resistant MDD received a single infusion of either AZD6765 (150 mg) or placebo on 2 test days 1 week apart. The primary outcome measure was the Montgomery-Åsberg Depression Rating Scale, which was used to rate overall depressive symptoms at baseline and 60, 80, 110, and 230 min postinfusion and on Days 1, 2, 3, and 7 postinfusion. Several secondary outcome measures were also used, including the Hamilton Depression Rating Scale.
Results: Within 80 min, Montgomery-Åsberg Depression Rating Scale scores significantly improved in subjects receiving AZD6765 compared with placebo; this improvement remained significant only through 110 min (d = .40). On the Hamilton Depression Rating Scale, a drug difference was found at 80 and 110 min and at Day 2 (d = .49). Overall, 32% of subjects responded to AZD6765, and 15% responded to placebo at some point during the trial. No difference was observed between the groups with regard to psychotomimetic or dissociative adverse effects.
Conclusions: In patients with treatment-resistant MDD, a single intravenous dose of the low-trapping NMDA channel blocker AZD6765 was associated with rapid but short-lived antidepressant effects; no psychotomimetic effects were observed.
Trial registration: ClinicalTrials.gov NCT00986479.
Keywords: Antidepressant; depression; glutamate; low-trapping NMDA; rapid-acting; treatment-resistant.
Published by Elsevier Inc.
Figures

Comment in
-
Exploiting N-methyl-d-aspartate channel blockade for a rapid antidepressant response in major depressive disorder.Biol Psychiatry. 2013 Aug 15;74(4):238-9. doi: 10.1016/j.biopsych.2013.05.029. Biol Psychiatry. 2013. PMID: 23885752 No abstract available.
References
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. - PubMed
-
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

