Rotigotine transdermal system for long-term treatment of patients with advanced Parkinson's disease: results of two open-label extension studies, CLEOPATRA-PD and PREFER
- PMID: 23208198
- PMCID: PMC3687107
- DOI: 10.1007/s00702-012-0925-5
Rotigotine transdermal system for long-term treatment of patients with advanced Parkinson's disease: results of two open-label extension studies, CLEOPATRA-PD and PREFER
Abstract
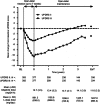
Open-label extensions [studies SP516 (NCT00501969) and SP715 (NCT00594386)] of the CLEOPATRA-PD and PREFER studies were conducted to evaluate the safety, tolerability and efficacy of the dopaminergic agonist, rotigotine, over several years of follow-up in patients with advanced Parkinson's disease (PD). Eligible subjects completing the double-blind trials received open-label adjunctive rotigotine (≤16 mg/24 h) for up to 4 and 6 years in Studies SP516 and SP715, respectively. Safety and tolerability were assessed using adverse events, vital signs and laboratory parameters, and efficacy assessed using the unified Parkinson's disease rating scale (UPDRS). Of the 395 and 258 patients enrolled in the SP516 and SP715 studies, 48 and 45 % completed, respectively. Adverse events were typically dopaminergic effects [e.g., somnolence (18-25 %/patient-year), insomnia (5-7 %/patient-year), dyskinesias (4-8 %/patient-year) and hallucinations (4-8 %/patient-year)], or related to the transdermal application of a patch (application site reactions: 14-15 %/patient-year). There were no clinically relevant changes in vital signs or laboratory parameters in either study. Mean UPDRS part II (activities of daily living) and part III (motor function) total scores improved from double-blind baseline during dose titration, then gradually declined over the maintenance period. In study SP516, mean UPDRS part II and III total scores were 0.8 points above and 2.8 points below double-blind baseline, respectively, at end of treatment. In study SP715, mean UPDRS part II and III total scores were 4.1 points above and 0.2 points below baseline, respectively, at end of treatment. In these open-label studies, adjunctive rotigotine was efficacious with an acceptable safety and tolerability profile in patients with advanced PD for up to 6 years.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

