Prions, prionoids and pathogenic proteins in Alzheimer disease
- PMID: 23208281
- PMCID: PMC3609051
- DOI: 10.4161/pri.23061
Prions, prionoids and pathogenic proteins in Alzheimer disease
Abstract
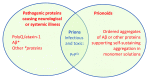
Like patients with prion disease, Alzheimer patients suffer from a fatal, progressive form of dementia. There is growing evidence that amyloid-β (Aβ) aggregates may be transmissible similar to prions, at least under extreme experimental conditions. However, unlike mice infected with prion protein (PrP) prions, those inoculated with Aβ do not die. The transmission of Aβ and PrP thus differs conspicuously in the neurological effects they induce in their hosts, the difference being no less than a matter of life and death. Far from being a mere academic nuance, this distinction between Aβ and PrP begs the crucial questions of what, exactly, controls prion toxicity and how prion toxicity relates to prion infectivity.
Keywords: Alzheimer’s disease; PrP; amyloid-β; pathogenic proteins; prionoids; prions; tau.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
