The spectrum of disease in chronic traumatic encephalopathy
- PMID: 23208308
- PMCID: PMC3624697
- DOI: 10.1093/brain/aws307
The spectrum of disease in chronic traumatic encephalopathy
Erratum in
- Brain. 2013 Oct;136(Pt 10):e255
Abstract
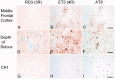
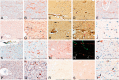
Chronic traumatic encephalopathy is a progressive tauopathy that occurs as a consequence of repetitive mild traumatic brain injury. We analysed post-mortem brains obtained from a cohort of 85 subjects with histories of repetitive mild traumatic brain injury and found evidence of chronic traumatic encephalopathy in 68 subjects: all males, ranging in age from 17 to 98 years (mean 59.5 years), including 64 athletes, 21 military veterans (86% of whom were also athletes) and one individual who engaged in self-injurious head banging behaviour. Eighteen age- and gender-matched individuals without a history of repetitive mild traumatic brain injury served as control subjects. In chronic traumatic encephalopathy, the spectrum of hyperphosphorylated tau pathology ranged in severity from focal perivascular epicentres of neurofibrillary tangles in the frontal neocortex to severe tauopathy affecting widespread brain regions, including the medial temporal lobe, thereby allowing a progressive staging of pathology from stages I-IV. Multifocal axonal varicosities and axonal loss were found in deep cortex and subcortical white matter at all stages of chronic traumatic encephalopathy. TAR DNA-binding protein 43 immunoreactive inclusions and neurites were also found in 85% of cases, ranging from focal pathology in stages I-III to widespread inclusions and neurites in stage IV. Symptoms in stage I chronic traumatic encephalopathy included headache and loss of attention and concentration. Additional symptoms in stage II included depression, explosivity and short-term memory loss. In stage III, executive dysfunction and cognitive impairment were found, and in stage IV, dementia, word-finding difficulty and aggression were characteristic. Data on athletic exposure were available for 34 American football players; the stage of chronic traumatic encephalopathy correlated with increased duration of football play, survival after football and age at death. Chronic traumatic encephalopathy was the sole diagnosis in 43 cases (63%); eight were also diagnosed with motor neuron disease (12%), seven with Alzheimer's disease (11%), 11 with Lewy body disease (16%) and four with frontotemporal lobar degeneration (6%). There is an ordered and predictable progression of hyperphosphorylated tau abnormalities through the nervous system in chronic traumatic encephalopathy that occurs in conjunction with widespread axonal disruption and loss. The frequent association of chronic traumatic encephalopathy with other neurodegenerative disorders suggests that repetitive brain trauma and hyperphosphorylated tau protein deposition promote the accumulation of other abnormally aggregated proteins including TAR DNA-binding protein 43, amyloid beta protein and alpha-synuclein.
Figures




References
-
- Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, et al. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133:3685–98. - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–9. - PubMed
-
- Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;2:244–54. - PubMed

