Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis
- PMID: 23208317
- PMCID: PMC7091913
- DOI: 10.1038/bmt.2012.241
Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis
Abstract
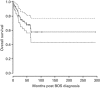
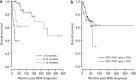
Bronchiolitis obliterans syndrome (BOS) after allogeneic hematopoietic SCT (HSCT) is recognized as a new-onset obstructive lung defect (OLD) in pulmonary function testing and is related to pulmonary chronic GVHD. Little is known about the different phenotypes of patients with BOS and their outcomes. We reviewed the data of all allogeneic HSCT recipients referred to our pulmonary department for a non-infectious bronchial disease between 1999 and 2010. We identified 103 patients (BOS (n=77), asthma (n=11) and chronic bronchitis (n=15)). In patients with BOS, we identified two functional phenotypes: a typical OLD, that is, forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio <0.7 (n=53), and an atypical OLD with a concomitant decrease in the FEV1 <80% and FVC <80% predicted with a normal total lung capacity (n=24). The typical OLD was characterized by more severe FEV1 and fewer centrilobular nodules on the computed tomography scan. The FEV1 was not significantly affected during the follow-up, regardless of the phenotype. In addition to acute and extensive chronic GVHD, only the occurrence of BOS soon after transplantation and the intentional treatment of BOS with steroids were associated with a poor survival. The determination of patient subgroups should be explored to improve the management of this condition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bergeron A, Chevret S, Chagnon K, Petropoulou A, Robin M, Desseaux K. Prospective evaluation of the incidence, risk factors and clinical outcome of late onset non infectious pulmonary complications after allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2011;183:4667.
-
- Filipovich AH, Weisdorf D, Pavletic S, Socié G, Wingard JR, Lee SJ. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

