A randomized, double-blind, controlled trial of the 17D yellow fever virus vaccine given in combination with immune globulin or placebo: comparative viremia and immunogenicity
- PMID: 23208880
- PMCID: PMC3541731
- DOI: 10.4269/ajtmh.2012.12-0179
A randomized, double-blind, controlled trial of the 17D yellow fever virus vaccine given in combination with immune globulin or placebo: comparative viremia and immunogenicity
Abstract
We evaluated whether coadministration of the yellow fever (YF) virus vaccine with human immunoglobulin (Ig) that contained YF virus-neutralizing antibodies would reduce post-vaccination viremia without compromising immunogenicity and thus, potentially mitigate YF vaccine-associated adverse events. We randomized 80 participants to receive either YF vaccine and Ig or YF vaccine and saline placebo. Participants were followed for 91 days for safety and assessments of viremia and immunogenicity. There were no differences found between the two groups in the proportion of vaccinated participants who developed viremia, seroconversion, cluster of differentiation (CD)-8(+) and CD4(+) T-cell responses, and cytokine responses. These results argue against one putative explanation for the increased reporting of YF vaccine side effects in recent years (i.e., a change in travel clinic practice after 1996 when hepatitis A prophylaxis with vaccine replaced routine use of pre-travel Ig, thus potentially removing an incidental YF vaccine-attenuating effect of anti-YF virus antibodies present in Ig).
Trial registration: ClinicalTrials.gov NCT00254826.
Conflict of interest statement
Disclosure: D.T. was employed by the study sponsor, Sanofi Pasteur, at the time that the study was conducted. Seema Garg moved to Sanofi Pasteur after the study was completed. These statements are made in the interest of full disclosure and not because the authors consider their employment to be a conflict of interest.
Figures


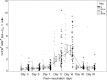
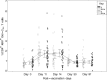
Comment in
-
17D Yellow Fever Virus Vaccine.Am J Trop Med Hyg. 2013 Dec;89(6):1225. doi: 10.4269/ajtmh.13-0443a. Am J Trop Med Hyg. 2013. PMID: 24306031 Free PMC article. No abstract available.
-
In response.Am J Trop Med Hyg. 2013 Dec;89(6):1226-1227. doi: 10.4269/ajtmh.13-0443b. Am J Trop Med Hyg. 2013. PMID: 24306032 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention . The Yellow Book. New York: Oxford University Press; 2012. Health information for international travel.
-
- Barrett ADT, Teuwen DE. Yellow fever vaccine—how does it work and why do rare cases of serious adverse events take place? Curr Opin Immunol. 2009;21:308–313. - PubMed
-
- Martin M, Tsai TF, Cropp B, Chang GJ, Holmes DA, Tseng J, Shieh W, Zaki SR, Al-Sanouri I, Cutrona AF, Ray G, Weld LH, Cetron MS. Fever and multiorgan failure associated with 17D-204 yellow fever: a report of four cases. Lancet. 2001;358:98–104. - PubMed
-
- Centers for Disease Control and Prevention (CDC) Adverse events associated with 17D-derived yellow fever vaccination—United States, 2001–2002. MMWR Morb Mortal Wkly Rep. 2002;51:989–993. - PubMed
-
- Pulendran B, Miller J, Querec TD, Akondy R, Moseley N, Laur O, Glidewell J, Monson N, Zhu T, Zhu H, Staprans S, Lee D, Brinton MA, Perelygin AA, Vellozzi C, Brachman P, Jr, Lalor S, Teuwen D, Eidex RB, Cetron M, Priddy F, del Rio C, Altman J, Ahmed R. Case of yellow fever vaccine-associated viscerotropic disease with prolonged viremia, robust adaptive immune responses, and polymorphisms in CCR5 and RANTES genes. J Infect Dis. 2008;198:500–507. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

