Wnt signaling in skin development, homeostasis, and disease
- PMID: 23209129
- PMCID: PMC3552514
- DOI: 10.1101/cshperspect.a008029
Wnt signaling in skin development, homeostasis, and disease
Abstract
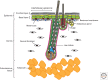
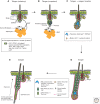
The skin and its appendages constitute the largest organ of the body. Its stratified epithelia offer protection from environmental stresses such as dehydration, irradiation, mechanical trauma, and pathogenic infection, whereas its appendages, like hair and sebaceous glands, help regulate body temperature as well as influence animal interaction and social behavior through camouflage and sexual signaling. To respond to and function effectively in a dynamic external environment, the skin and its appendages possess a remarkable ability to regenerate in a carefully controlled fashion. When this finely tuned homeostatic process is disrupted, skin diseases such as cancers may result. At present, the molecular signals that orchestrate cell proliferation, differentiation, and patterning in the skin remain incompletely understood. It is increasingly apparent that many morphogenetic pathways with key roles in development are also important in regulating skin biology. Of these, Wnt signaling has emerged as the dominant pathway controlling the patterning of skin and influencing the decisions of embryonic and adult stem cells to adopt the various cell lineages of the skin and its appendages, as well as subsequently controlling the function of differentiated skin cells. Here we will review established concepts and present recent advances in our understanding of the diverse roles that Wnt signaling plays in skin development, homeostasis, and disease.
Figures


References
-
- Ambler CA, Määttä A 2009. Epidermal stem cells: Location, potential and contribution to cancer. J Pathol 217: 206–216 - PubMed
-
- Andl T, Reddy ST, Gaddapara T, Millar SE 2002. WNT signals are required for the initiation of hair follicle development. Dev Cell 2: 643–653 - PubMed
-
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. 2004. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131: 2257–2268 - PubMed
-
- Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA 2006. β-Catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 296: 164–176 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
