Phenotypic plasticity in evolutionary rescue experiments
- PMID: 23209170
- PMCID: PMC3538455
- DOI: 10.1098/rstb.2012.0089
Phenotypic plasticity in evolutionary rescue experiments
Abstract
Population persistence in a new and stressful environment can be influenced by the plastic phenotypic responses of individuals to this environment, and by the genetic evolution of plasticity itself. This process has recently been investigated theoretically, but testing the quantitative predictions in the wild is challenging because (i) there are usually not enough population replicates to deal with the stochasticity of the evolutionary process, (ii) environmental conditions are not controlled, and (iii) measuring selection and the inheritance of traits affecting fitness is difficult in natural populations. As an alternative, predictions from theory can be tested in the laboratory with controlled experiments. To illustrate the feasibility of this approach, we briefly review the literature on the experimental evolution of plasticity, and on evolutionary rescue in the laboratory, paying particular attention to differences and similarities between microbes and multicellular eukaryotes. We then highlight a set of questions that could be addressed using this framework, which would enable testing the robustness of theoretical predictions, and provide new insights into areas that have received little theoretical attention to date.
Figures


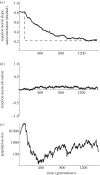
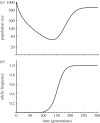
References
-
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) - DOI
-
- Davis MB, Shaw RG, Etterson JR. 2005. Evolutionary responses to changing climate. Ecology 86, 1704–1714 10.1890/03-0788 (doi:10.1890/03-0788) - DOI
-
- Caswell H. 2001. Matrix population models: construction, analysis, and interpretation. Sunderland, MA: Sinauer Associates
-
- Charlesworth B. 1994. Evolution in age-structured populations, 2nd edn Cambridge, UK: Cambridge University Press
-
- Gomulkiewicz R, Holt RD. 1995. When does evolution by natural selection prevent extinction. Evolution 49, 201–207 10.2307/2410305 (doi:10.2307/2410305) - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
