Human immunodeficiency virus vaccine trials
- PMID: 23209178
- PMCID: PMC3543076
- DOI: 10.1101/cshperspect.a007351
Human immunodeficiency virus vaccine trials
Abstract
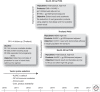
More than 2 million AIDS-related deaths occurred globally in 2008, and more than 33 million people are living with HIV/AIDS. Despite promising advances in prevention, an estimated 2.7 million new HIV infections occurred in that year, so that for every two patients placed on combination antiretroviral treatment, five people became infected. The pandemic poses a formidable challenge to the development, progress, and stability of global society 30 years after it was recognized. Experimental preventive HIV-1 vaccines have been administered to more than 44,000 human volunteers in more than 187 separate trials since 1987. Only five candidate vaccine strategies have been advanced to efficacy testing. The recombinant glycoprotein (rgp)120 subunit vaccines, AIDSVAX B/B and AIDSVAX B/E, and the Merck Adenovirus serotype (Ad)5 viral-vector expressing HIV-1 Gag, Pol, and Nef failed to show a reduction in infection rate or lowering of postinfection viral set point. Most recently, a phase III trial that tested a heterologous prime-boost vaccine combination of ALVAC-HIV vCP1521 and bivalent rgp120 (AIDSVAX B/E) showed 31% efficacy in protection from infection among community-risk Thai participants. A fifth efficacy trial testing a DNA/recombinant(r) Ad5 prime-boost combination is currently under way. We review the clinical trials of HIV vaccines that have provided insight into human immunogenicity or efficacy in preventing HIV-1 infection.
Figures
References
-
- Abdulhaqq SA, Weiner DB 2008. DNA vaccines: Developing new strategies to enhance immune responses. Immunol Res 42: 219–232 - PubMed
-
- Abrahams MR, Anderson JA, Giorgi EE, Seoighe C, Mlisana K, Ping LH, Athreya GS, Treurnicht FK, Keele BF, Wood N, et al. 2009. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J Virol 83: 3556–3567 - PMC - PubMed
-
- Allen TM, Vogel TU, Fuller DH, Mothé BR, Steffen S, Boyson JE, Shipley T, Fuller J, Hanke T, Sette A, et al. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J Immunol 164: 4968–4978 - PubMed
-
- Asmuth DM, Brown EL, DiNubile MJ, Sun X, del Rio C, Harro C, Keefer MC, Kublin JG, Dubey SA, Kierstead LS, et al. 2010. Comparative cell-mediated immunogenicity of DNA/DNA, DNA/adenovirus type 5 (Ad5), or Ad5/Ad5 HIV-1 clade B gag vaccine prime-boost regimens. J Infect Dis 201: 132–141 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
