Functional requirement for a highly conserved charged residue at position 75 in the gap junction protein connexin 32
- PMID: 23209285
- PMCID: PMC3561579
- DOI: 10.1074/jbc.M112.392670
Functional requirement for a highly conserved charged residue at position 75 in the gap junction protein connexin 32
Abstract
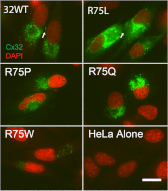
Charcot Marie Tooth disease (CMT) is a group of inherited disorders characterized clinically by exclusively or predominantly peripheral nerve dysfunction. CMT1X, the most common form of X-linked CMT is caused by mutations in connexin 32 (Cx32). In this work, we used dual whole cell patch clamp recording to examine the functional effects of mutations at the Arg(75) position. This residue is highly conserved among members of the connexin family, and disease-causing mutations have been identified at this (or the corresponding) position in Cx26, Cx43, and Cx46. Thus, a better understanding of the effects of mutations of this position in Cx32 may have relevance to pathogenesis of a number of different human diseases. All three mutants associated with CMT1X (R75P, R75Q, and R75W) showed very low levels of coupling similar to those of the cells transfected with vector alone. Heterotypic pairing with Cx32 WT showed that the absence of coupling for these mutants in the homotypic configuration could be explained by shifts in their hemichannel G(j)-V(j) relations. Examination of the expression levels and gating characteristics of seven additional mutants (R75A, R75D, R75E, R75H, R75K, R75L, and R75V) at this position suggest that the positive charge at position 75 in Cx32 is required for normal channel function but not for gap junction assembly. Our studies also suggest that disease treatment strategies for CMT1X, which correct trafficking abnormalities in Cx32, may be ineffective for the group of mutations also conferring changes in gating properties of Cx32 channels.
Figures




References
-
- Willecke K., Eiberger J., Degen J., Eckardt D., Romualdi A., Güldenagel M., Deutsch U., Söhl G. (2002) Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 383, 725–737 - PubMed
-
- Oh S., Ri Y., Bennett M. V., Trexler E. B., Verselis V. K., Bargiello T. A. (1997) Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron 19, 927–938 - PubMed
-
- Kumar N. M., Gilula N. B. (1996) The gap junction communication channel. Cell 84, 381–388 - PubMed
-
- Gaietta G., Deerinck T. J., Adams S. R., Bouwer J., Tour O., Laird D. W., Sosinsky G. E., Tsien R. Y., Ellisman M. H. (2002) Multicolor and electron microscopic imaging of connexin trafficking. Science 296, 503–507 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

