The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells
- PMID: 23209291
- PMCID: PMC3542994
- DOI: 10.1074/jbc.M112.393090
The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells
Abstract
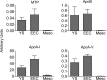
During chicken yolk sac (YS) growth, mesodermal cells in the area vasculosa follow the migrating endodermal epithelial cell (EEC) layer in the area vitellina. Ultimately, these cells form the vascularized YS that functions in nutrient transfer to the embryo. How and when EECs, with their apical aspect directly contacting the oocytic yolk, acquire the ability to take up yolk macromolecules during the vitellina-to-vasculosa transition has not been investigated. In addressing these questions, we found that with progressive vascularization, the expression level in EECs of the nutrient receptor triad, LRP2-cubilin-amnionless, changes significantly. The receptor complex, competent for uptake of yolk proteins, is produced by EECs in the area vasculosa but not in the area vitellina. Yolk components endocytosed by LRP2-cubilin-amnionless, preformed and newly formed lipid droplets, and yolk-derived very low density lipoprotein, shown to be efficiently endocytosed and lysosomally processed by EECs, probably provide substrates for resynthesis and secretion of nutrients, such as lipoproteins. In fact, as directly demonstrated by pulse-chase experiments, EECs in the vascularized, but not in the avascular, region efficiently produce and secrete lipoproteins containing apolipoprotein A-I (apoA-I), apoB, and/or apoA-V. In contrast, perilipin 2, a lipid droplet-stabilizing protein, is produced exclusively by the EECs of the area vitellina. These data suggest a differentiation process that orchestrates the vascularization of the developing YS with the induction of yolk uptake and lipoprotein secretion by EECs to ensure embryo nutrition.
Figures








References
-
- Zohn I. E., Sarkar A. A. (2010) The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birth Defects Res. A Clin. Mol. Teratol. 88, 593–600 - PubMed
-
- Schneider W. J., Osanger A., Waclawek M., Nimpf J. (1998) Oocyte growth in the chicken. Receptors and more. Biol. Chem. 379, 965–971 - PubMed
-
- Palis J., McGrath K. E., Kingsley P. D. (1995) Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood 86, 156–163 - PubMed
-
- Yoshizaki N., Soga M., Ito Y., Mao K. M., Sultana F., Yonezawa S. (2004) Two-step consumption of yolk granules during the development of quail embryos. Dev. Growth Differ. 46, 229–238 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

