Anatomy and histology of rodent and human major salivary glands: -overview of the Japan salivary gland society-sponsored workshop-
- PMID: 23209333
- PMCID: PMC3496860
- DOI: 10.1267/ahc.12013
Anatomy and histology of rodent and human major salivary glands: -overview of the Japan salivary gland society-sponsored workshop-
Abstract
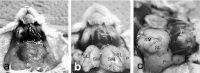
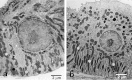
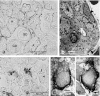
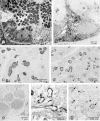
MAJOR SALIVARY GLANDS OF BOTH HUMANS AND RODENTS CONSIST OF THREE PAIRS OF MACROSCOPIC GLANDS: parotid, submandibular, and sublingual. These glands secrete serous, mucous or mixed saliva via the proper main excretory ducts connecting the glandular bodies with the oral cavity. A series of discoveries about the salivary ducts in the 17th century by Niels Stensen (1638-1686), Thomas Wharton (1614-1673), and Caspar Bartholin (1655-1738) established the concept of exocrine secretion as well as salivary glands. Recent investigations have revealed the endocrine functions of parotin and a variety of cell growth factors produced by salivary glands.The present review aims to describe macroscopic findings on the major salivary glands of rodents and the microscopic differences between those of humans and rodents, which review should be of interest to those researchers studying salivary glands.
Keywords: human; immunohistochemistry; mouse; rat; salivary glands.
Figures
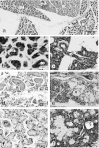
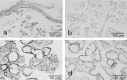
References
-
- Amano O. The salivary gland: anatomy for surgeons and researchers. Jpn. J. Oral Maxillofac. Surg. 2011;57:384–393.
-
- Amano O., Tsuji T., Nakamura T., Iseki S. Expression of transforming growth factor beta-1 in the submandibular gland of the rat. J. Histochem. Cytochem. 1991;39:1707–1711. - PubMed
-
- Amano O., Iseki S. Expression, localization and developmental regulation of insulin-like growth factor I mRNA in rat submandibular gland. Archs. Oral Biol. 1993;38:671–677. - PubMed
-
- Amano O., Yoshitake Y., Nishikawa K., Iseki S. Basic fibroblast growth factor in rat salivary glands. Cell Tissue Res. 1993;273:467–474. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

