Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host
- PMID: 23209379
- PMCID: PMC3507920
- DOI: 10.1371/journal.pbio.1001435
Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host
Abstract
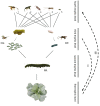
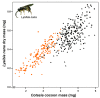
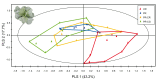
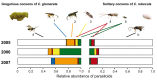
Plants respond to herbivory with the emission of induced plant volatiles. These volatiles may attract parasitic wasps (parasitoids) that attack the herbivores. Although in this sense the emission of volatiles has been hypothesized to be beneficial to the plant, it is still debated whether this is also the case under natural conditions because other organisms such as herbivores also respond to the emitted volatiles. One important group of organisms, the enemies of parasitoids, hyperparasitoids, has not been included in this debate because little is known about their foraging behaviour. Here, we address whether hyperparasitoids use herbivore-induced plant volatiles to locate their host. We show that hyperparasitoids find their victims through herbivore-induced plant volatiles emitted in response to attack by caterpillars that in turn had been parasitized by primary parasitoids. Moreover, only one of two species of parasitoids affected herbivore-induced plant volatiles resulting in the attraction of more hyperparasitoids than volatiles from plants damaged by healthy caterpillars. This resulted in higher levels of hyperparasitism of the parasitoid that indirectly gave away its presence through its effect on plant odours induced by its caterpillar host. Here, we provide evidence for a role of compounds in the oral secretion of parasitized caterpillars that induce these changes in plant volatile emission. Our results demonstrate that the effects of herbivore-induced plant volatiles should be placed in a community-wide perspective that includes species in the fourth trophic level to improve our understanding of the ecological functions of volatile release by plants. Furthermore, these findings suggest that the impact of species in the fourth trophic level should also be considered when developing Integrated Pest Management strategies aimed at optimizing the control of insect pests using parasitoids.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
Trophic complexity and the adaptive value of damage-induced plant volatiles.PLoS Biol. 2012;10(11):e1001437. doi: 10.1371/journal.pbio.1001437. Epub 2012 Nov 27. PLoS Biol. 2012. PMID: 23209381 Free PMC article.
References
-
- Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37: 141–172.
-
- Hare JD (2011) Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol 56: 161–180. - PubMed
-
- Kessler A, Heil M (2011) The multiple faces of indirect defences and their agents of natural selection. Funct Ecol 25: 348–357.
-
- Price PW, Bouton CE, Gross P, Mcpheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11: 41–65.
-
- Dicke M, van Loon JJA, Soler R (2009) Chemical complexity of volatiles from plants induced by multiple attack. Nat Chem Biol 5: 317–324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

