Epigenetics of host-pathogen interactions: the road ahead and the road behind
- PMID: 23209403
- PMCID: PMC3510240
- DOI: 10.1371/journal.ppat.1003007
Epigenetics of host-pathogen interactions: the road ahead and the road behind
Abstract
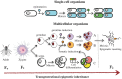
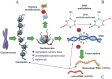
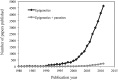
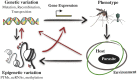
A growing body of evidence points towards epigenetic mechanisms being responsible for a wide range of biological phenomena, from the plasticity of plant growth and development to the nutritional control of caste determination in honeybees and the etiology of human disease (e.g., cancer). With the (partial) elucidation of the molecular basis of epigenetic variation and the heritability of certain of these changes, the field of evolutionary epigenetics is flourishing. Despite this, the role of epigenetics in shaping host-pathogen interactions has received comparatively little attention. Yet there is plenty of evidence supporting the implication of epigenetic mechanisms in the modulation of the biological interaction between hosts and pathogens. The phenotypic plasticity of many key parasite life-history traits appears to be under epigenetic control. Moreover, pathogen-induced effects in host phenotype may have transgenerational consequences, and the bases of these changes and their heritability probably have an epigenetic component. The significance of epigenetic modifications may, however, go beyond providing a mechanistic basis for host and pathogen plasticity. Epigenetic epidemiology has recently emerged as a promising area for future research on infectious diseases. In addition, the incorporation of epigenetic inheritance and epigenetic plasticity mechanisms to evolutionary models and empirical studies of host-pathogen interactions will provide new insights into the evolution and coevolution of these associations. Here, we review the evidence available for the role epigenetics on host-pathogen interactions, and the utility and versatility of the epigenetic technologies available that can be cross-applied to host-pathogen studies. We conclude with recommendations and directions for future research on the burgeoning field of epigenetics as applied to host-pathogen interactions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Waddington CH (1942) The epigenome. Endevour 1: 18–20.
-
- Jablonka E, Lamb MJ (2002) The changing concept of epigenetics. In: VanSpeybroeck L, VandeVijver G, DeWaele D, editors. From epigenesis to epigenetics: the genome in context. New York: New York Academy of Sciences. pp. 82–96. - PubMed
-
- Suzuki MM, Bird A (2008) DNA methylation landscapes: provocative insights from epigenomics. Nature Reviews in Genetics 9: 465–476. - PubMed
-
- Storz G (2002) An expanding universe of noncoding RNAs. Science 296: 1260–1263. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

