RUNX3, EGR1 and SOX9B form a regulatory cascade required to modulate BMP-signaling during cranial cartilage development in zebrafish
- PMID: 23209659
- PMCID: PMC3507947
- DOI: 10.1371/journal.pone.0050140
RUNX3, EGR1 and SOX9B form a regulatory cascade required to modulate BMP-signaling during cranial cartilage development in zebrafish
Abstract
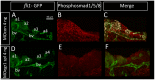
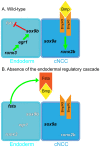
The cartilaginous elements forming the pharyngeal arches of the zebrafish derive from cranial neural crest cells. Their proper differentiation and patterning are regulated by reciprocal interactions between neural crest cells and surrounding endodermal, ectodermal and mesodermal tissues. In this study, we show that the endodermal factors Runx3 and Sox9b form a regulatory cascade with Egr1 resulting in transcriptional repression of the fsta gene, encoding a BMP antagonist, in pharyngeal endoderm. Using a transgenic line expressing a dominant negative BMP receptor or a specific BMP inhibitor (dorsomorphin), we show that BMP signaling is indeed required around 30 hpf in the neural crest cells to allow cell differentiation and proper pharyngeal cartilage formation. Runx3, Egr1, Sox9b and BMP signaling are required for expression of runx2b, one of the key regulator of cranial cartilage maturation and bone formation. Finally, we show that egr1 depletion leads to increased expression of fsta and inhibition of BMP signaling in the pharyngeal region. In conclusion, we show that the successive induction of the transcription factors Runx3, Egr1 and Sox9b constitutes a regulatory cascade that controls expression of Follistatin A in pharyngeal endoderm, the latter modulating BMP signaling in developing cranial cartilage in zebrafish.
Conflict of interest statement
Figures





Similar articles
-
Fgf receptors Fgfr1a and Fgfr2 control the function of pharyngeal endoderm in late cranial cartilage development.Differentiation. 2013 Nov-Dec;86(4-5):192-206. doi: 10.1016/j.diff.2013.07.006. Epub 2013 Oct 29. Differentiation. 2013. PMID: 24176552
-
An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning.Development. 2004 Nov;131(22):5703-16. doi: 10.1242/dev.01444. Development. 2004. PMID: 15509770
-
Tgfbeta3 regulation of chondrogenesis and osteogenesis in zebrafish is mediated through formation and survival of a subpopulation of the cranial neural crest.Mech Dev. 2010 Jul-Aug;127(7-8):329-44. doi: 10.1016/j.mod.2010.04.003. Epub 2010 Apr 18. Mech Dev. 2010. PMID: 20406684
-
Pathways in blood and vessel development revealed through zebrafish genetics.Int J Dev Biol. 2002;46(4):493-502. Int J Dev Biol. 2002. PMID: 12141436 Review.
-
From Endoderm to Progenitors: An Update on the Early Steps of Thyroid Morphogenesis in the Zebrafish.Front Endocrinol (Lausanne). 2021 Jun 4;12:664557. doi: 10.3389/fendo.2021.664557. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34149617 Free PMC article. Review.
Cited by
-
Globularity and language-readiness: generating new predictions by expanding the set of genes of interest.Front Psychol. 2014 Nov 25;5:1324. doi: 10.3389/fpsyg.2014.01324. eCollection 2014. Front Psychol. 2014. PMID: 25505436 Free PMC article.
-
Transgenic fluorescent zebrafish lines that have revolutionized biomedical research.Lab Anim Res. 2021 Sep 8;37(1):26. doi: 10.1186/s42826-021-00103-2. Lab Anim Res. 2021. PMID: 34496973 Free PMC article. Review.
-
Dosage analysis of the 7q11.23 Williams region identifies BAZ1B as a major human gene patterning the modern human face and underlying self-domestication.Sci Adv. 2019 Dec 4;5(12):eaaw7908. doi: 10.1126/sciadv.aaw7908. eCollection 2019 Dec. Sci Adv. 2019. PMID: 31840056 Free PMC article.
-
Testing biological actions of medicinal plants from northern Vietnam on zebrafish embryos and larvae: Developmental, behavioral, and putative therapeutical effects.PLoS One. 2023 Nov 7;18(11):e0294048. doi: 10.1371/journal.pone.0294048. eCollection 2023. PLoS One. 2023. PMID: 37934745 Free PMC article.
-
Protein-protein interaction network module changes associated with the vertebrate fin-to-limb transition.Sci Rep. 2023 Dec 18;13(1):22594. doi: 10.1038/s41598-023-50050-2. Sci Rep. 2023. PMID: 38114646 Free PMC article.
References
-
- Schilling TF, Kimmel CB (1994) Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 120: 483–494. - PubMed
-
- Knight RD, Javidan Y, Zhang T, Nelson S, Schilling TF (2005) AP2-dependent signals from the ectoderm regulate craniofacial development in the zebrafish embryo. Development 132: 3127–3138. - PubMed
-
- Yanagisawa H, Clouthier DE, Richardson JA, Charite J, Olson EN (2003) Targeted deletion of a branchial arch-specific enhancer reveals a role of dHAND in craniofacial development. Development 130: 1069–1078. - PubMed
-
- Sperber SM, Saxena V, Hatch G, Ekker M (2008) Zebrafish dlx2a contributes to hindbrain neural crest survival, is necessary for differentiation of sensory ganglia and functions with dlx1a in maturation of the arch cartilage elements. Dev Biol 314: 59–70. - PubMed
-
- Yan YL, Willoughby J, Liu D, Crump JG, Wilson C, et al. (2005) A pair of Sox: distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development 132: 1069–1083. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

