Infectious complications in head and neck cancer patients treated with cetuximab: propensity score and instrumental variable analysis
- PMID: 23209663
- PMCID: PMC3509146
- DOI: 10.1371/journal.pone.0050163
Infectious complications in head and neck cancer patients treated with cetuximab: propensity score and instrumental variable analysis
Abstract
Background: To compare the infection rates between cetuximab-treated patients with head and neck cancers (HNC) and untreated patients.
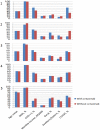
Methodology: A national cohort of 1083 HNC patients identified in 2010 from the Taiwan National Health Insurance Research Database was established. After patients were followed for one year, propensity score analysis and instrumental variable analysis were performed to assess the association between cetuximab therapy and the infection rates.
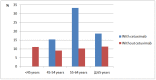
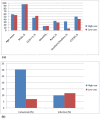
Results: HNC patients receiving cetuximab (n = 158) were older, had lower SES, and resided more frequently in rural areas as compared to those without cetuximab therapy. 125 patients, 32 (20.3%) in the group using cetuximab and 93 (10.1%) in the group not using it presented infections. The propensity score analysis revealed a 2.3-fold (adjusted odds ratio [OR] = 2.27; 95% CI, 1.46-3.54; P = 0.001) increased risk for infection in HNC patients treated with cetuximab. However, using IVA, the average treatment effect of cetuximab was not statistically associated with increased risk of infection (OR, 0.87; 95% CI, 0.61-1.14).
Conclusions: Cetuximab therapy was not statistically associated with infection rate in HNC patients. However, older HNC patients using cetuximab may incur up to 33% infection rate during one year. Particular attention should be given to older HNC patients treated with cetuximab.
Conflict of interest statement
Figures
Similar articles
-
Bloodstream infection in patients with head and neck cancer: a major challenge in the cetuximab era.Clin Transl Oncol. 2019 Feb;21(2):187-196. doi: 10.1007/s12094-018-1905-5. Epub 2018 Jun 11. Clin Transl Oncol. 2019. PMID: 29948973
-
Monoclonal antibodies targeting epidermal growth factor receptor and vascular endothelial growth factor with a focus on head and neck tumors.Curr Opin Oncol. 2005 May;17(3):212-7. doi: 10.1097/01.cco.0000159623.68506.cf. Curr Opin Oncol. 2005. PMID: 15818163 Review.
-
Cost effectiveness of cetuximab concurrent with radiotherapy for patients with locally advanced head and neck cancer in Taiwan: a decision-tree analysis.Clin Drug Investig. 2011 Oct 1;31(10):717-26. doi: 10.2165/11588980-000000000-00000. Clin Drug Investig. 2011. PMID: 21744880
-
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck.N Engl J Med. 2006 Feb 9;354(6):567-78. doi: 10.1056/NEJMoa053422. N Engl J Med. 2006. PMID: 16467544 Clinical Trial.
-
The role of cetuximab in the treatment of squamous cell cancer of the head and neck.Expert Opin Biol Ther. 2005 Aug;5(8):1085-93. doi: 10.1517/14712598.5.8.1085. Expert Opin Biol Ther. 2005. PMID: 16050785 Review.
Cited by
-
High incidence of ischemic stroke occurrence in irradiated lung cancer patients: a population-based surgical cohort study.PLoS One. 2014 Apr 7;9(4):e94377. doi: 10.1371/journal.pone.0094377. eCollection 2014. PLoS One. 2014. PMID: 24709837 Free PMC article.
-
Irradiation enhanced risks of hospitalised pneumonopathy in lung cancer patients: a population-based surgical cohort study.BMJ Open. 2017 Sep 27;7(9):e015022. doi: 10.1136/bmjopen-2016-015022. BMJ Open. 2017. PMID: 28963281 Free PMC article.
-
Incidence and risk of severe infections associated with anti-epidermal growth factor receptor monoclonal antibodies in cancer patients: a systematic review and meta-analysis.BMC Med. 2014 Nov 5;12:203. doi: 10.1186/s12916-014-0203-5. BMC Med. 2014. PMID: 25369798 Free PMC article.
-
Association between phosphorylated AMP-activated protein kinase and acetyl-CoA carboxylase expression and outcome in patients with squamous cell carcinoma of the head and neck.PLoS One. 2014 Apr 25;9(4):e96183. doi: 10.1371/journal.pone.0096183. eCollection 2014. PLoS One. 2014. PMID: 24769813 Free PMC article.
-
Bloodstream infection in patients with head and neck cancer: a major challenge in the cetuximab era.Clin Transl Oncol. 2019 Feb;21(2):187-196. doi: 10.1007/s12094-018-1905-5. Epub 2018 Jun 11. Clin Transl Oncol. 2019. PMID: 29948973
References
-
- Bernier J (2008) Drug Insight: cetuximab in the treatment of recurrent and metastatic squamous cell carcinoma of the head and neck. Nat Clin Prac Oncol 5: 705–713. - PubMed
-
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, et al. (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354: 567–578. - PubMed
-
- Vermorken J, Mesia R, Rivera F, Remenar E, Kawecki A, et al. (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359: 1116–1127. - PubMed
-
- Merlano M, Russi E, Benasso M, Corvò R, Colantonio I, et al. (2011) Cisplatin-based chemoradiation plus cetuximab in locally advanced head and neck cancer: a phase II clinical study. Annals of Oncology 22: 712–717. - PubMed
-
- Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, et al. (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. The Lancet Oncology 11: 21–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

