Placebo cohorts in phase-3 MS treatment trials - predictors for on-trial disease activity 1990-2010 based on a meta-analysis and individual case data
- PMID: 23209717
- PMCID: PMC3510222
- DOI: 10.1371/journal.pone.0050347
Placebo cohorts in phase-3 MS treatment trials - predictors for on-trial disease activity 1990-2010 based on a meta-analysis and individual case data
Abstract
Background: Annualized relapse rates (ARR) in the placebo cohorts of phase-3 randomized controlled trials (RCT) of new treatments for relapsing remitting multiple sclerosis (RRMS) have decreased substantially during the last two decades. The causes of these changes are not clear. We consider a better understanding of this phenomenon essential for valuing the effects of new drugs and by designing new trials.
Objectives: To identify predictive factors of on-study ARR in early and recent MS trials.
Methods: ARR, rate of relapse-free patients, trial start dates, baseline demographics, relapse definitions and the use of McDonald criteria were retrieved by literature research of the placebo cohorts from RRMS phase-3 trials. Predictors were estimated by univariate and multivariate regression analyses and random-effects meta-regression. In addition, regression models were calculated by the Sylvia Lawry Centre's (SLC), including individual case data from clinical trials performed until 2000. The most reliable meta-analytic results can be gained from pooled individual case data. In lack of this, random-effects meta-analyses are recommended.
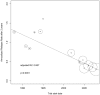
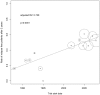
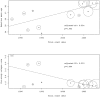
Results: Data from 12 published and one unpublished trial show a decrease of ARR from 1988 to 2012 (adjR(2) = 0.807, p<0.0001). Regression models identified McDonald criteria followed by baseline mean age and the pre-study relapse rate as predictors of the ARR. The pooled individual case data (n = 505) confirmed a decrease of ARR over time. The pre-study relapse rate was the best predictor for on-study relapses. Lacking individual case data after implementation of the McDonald criteria excludes a direct comparison concerning McDonald criteria.
Conclusion: Pre-study relapse rate was the best predictor for on-study relapse rate but failed to explain the decrease of the ARR over time alone. Higher age at baseline and the implementation of McDonald criteria were associated as well with a lowered relapse rate in the random-effects meta-regression. These findings need further clarification based on individual case data.
Conflict of interest statement
Figures
References
-
- Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, et al... (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. New England Journal of Medicine: 387–401. - PubMed
-
- Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, et al. (2010) A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. The New England journal of medicine 362: 416–426 doi:10.1056/NEJMoa0902533. - DOI - PubMed
-
- Jacobs LD, Cookfair DL, Rudick R a, Herndon RM, Richert JR, et al. (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Annals of neurology 39: 285–294 doi:10.1002/ana.410390304. - DOI - PubMed
-
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 352: 1498–1504 doi:10.1016/S0140-6736(98)03334–0. - DOI - PubMed
-
- Held U, Heigenhauser L, Shang C, Kappos L, Polman C (2005) Predictors of relapse rate in MS clinical trials. Neurology 65: 1769–1773 doi:10.1212/01.wnl.0000187122.71735.1f. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

