A single glycine-alanine exchange directs ligand specificity of the elephant progestin receptor
- PMID: 23209719
- PMCID: PMC3507690
- DOI: 10.1371/journal.pone.0050350
A single glycine-alanine exchange directs ligand specificity of the elephant progestin receptor
Abstract
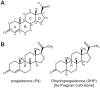
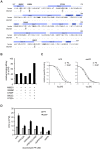
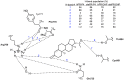
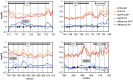
The primary gestagen of elephants is 5α-dihydroprogesterone (DHP), which is unlike all other mammals studied until now. The level of DHP in elephants equals that of progesterone in other mammals, and elephants are able to bind DHP with similar affinity to progesterone indicating a unique ligand-binding specificity of the elephant progestin receptor (PR). Using site-directed mutagenesis in combination with in vitro binding studies we here report that this change in specificity is due to a single glycine to alanine exchange at position 722 (G722A) of PR, which specifically increases DHP affinity while not affecting binding of progesterone. By conducting molecular dynamics simulations comparing human and elephant PR ligand-binding domains (LBD), we observed that the alanine methyl group at position 722 is able to push the DHP A-ring into a position similar to progesterone. In the human PR, the DHP A-ring position is twisted towards helix 3 of PR thereby disturbing the hydrogen bond pattern around the C3-keto group, resulting in a lower binding affinity. Furthermore, we observed that the elephant PR ligand-binding pocket is more rigid than the human analogue, which probably explains the higher affinity towards both progesterone and DHP. Interestingly, the G722A substitution is not elephant-specific, rather it is also present in five independent lineages of mammalian evolution, suggesting a special role of the substitution for the development of distinct mammalian gestagen systems.
Conflict of interest statement
Figures


References
-
- Mulac-Jericevic B, Conneely OM (2004) Reproductive tissue selective actions of progesterone receptors. Reproduction 128: 139–146 doi:10.1530/rep.1.00189 - DOI - PubMed
-
- Jewgenow K, Meyer HH (1998) Comparative binding affinity study of progestins to the cytosol progestin receptor of endometrium in different mammals. General and Comparative Endocrinology 110: 118–124 doi:10.1006/gcen.1997.7054 - DOI - PubMed
-
- Meyer HH, Jewgenow K, Hodges JK (1997) Binding activity of 5alpha-reduced gestagens to the progestin receptor from African elephant (Loxodonta africana). General and Comparative Endocrinology 105: 164–167 doi:10.1006/gcen.1996.6813 - DOI - PubMed
-
- Hodges JK, Heistermann M, Beard A, van Aarde RJ (1997) Concentrations of progesterone and the 5 alpha-reduced progestins, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one, in luteal tissue and circulating blood and their relationship to luteal function in the African elephant, Loxodonta africana. Biol Reprod 56: 640–646. - PubMed
-
- Hodges JK (1998) Endocrinology of the ovarian cycle and pregnancy in the Asian (Elephas maximus) and African (Loxodonta africana) elephant. Anim Reprod Sci 53: 3–18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

