Glutamine treatment attenuates endoplasmic reticulum stress and apoptosis in TNBS-induced colitis
- PMID: 23209735
- PMCID: PMC3508929
- DOI: 10.1371/journal.pone.0050407
Glutamine treatment attenuates endoplasmic reticulum stress and apoptosis in TNBS-induced colitis
Expression of concern in
-
Expression of Concern: Glutamine Treatment Attenuates Endoplasmic Reticulum Stress and Apoptosis in TNBS-Induced Colitis.PLoS One. 2024 Jan 19;19(1):e0297611. doi: 10.1371/journal.pone.0297611. eCollection 2024. PLoS One. 2024. PMID: 38241231 Free PMC article. No abstract available.
Abstract
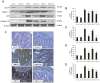
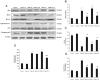
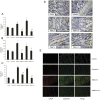
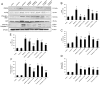
Endoplasmic reticulum (ER) stress and apoptotic cell death play an important role in the pathogenesis and perpetuation of inflammatory bowel disease (IBD). We aimed to explore the potential of glutamine to reduce ER stress and apoptosis in a rat model of experimental IBD. Colitis was induced in male Wistar rats by intracolonic administration of 30 mg of 2,4,6-trinitrobenzene sulfonic acid (TNBS). Glutamine (25 mg/dL) was given by rectal route daily for 2 d or 7 d. Both oxidative stress (TBARS concentration and oxidised/reduced glutathione ratio) and ER stress markers (CHOP, BiP, calpain-1 and caspase-12 expression) increased significantly within 48 h of TNBS instillation, and glutamine attenuated the extent of the changes. Glutamine also inhibited the significant increases of ATF6, ATF4 and spliced XBP-1 mRNA levels induced by TNBS instillation. TNBS-colitis resulted in a significant increase in p53 and cytochrome c expression, and a reduced Bcl-xL expression and Bax/Bcl-2 ratio. These effects were significantly inhibited by glutamine. Treatment with the amino acid also resulted in significant decreases of caspase-9, caspase-8 and caspase-3 activities. Double immunofluorescence staining showed co-localization of CHOP and cleaved caspase-3 in colon sections. Phospho-JNK and PARP-1 expression was also significantly higher in TNBS-treated rats, and treatment with glutamine significantly decreased JNK phosphorylation and PARP-1 proteolysis. To directly address the effect of glutamine on ER stress and apoptosis in epithelial cells, the ER stress inducers brefeldin A and tunicamycin were added to Caco-2 cells that were treated with glutamine (5 mM and 10 mM). The significant enhancement in PERK, ATF6 phosphorylated IRE1, BiP and cleaved caspase-3 expression induced by brefeldin A and tunicamycin was partly prevented by glutamine. Data obtained indicated that modulation of ER stress signalling and anti-apoptotic effects contribute to protection by glutamine against damage in TNBS-induced colitis.
Conflict of interest statement
Figures

References
-
- Fillmann H, Kretzmann NA, San-Miguel B, Llesuy S, Marroni N, et al. (2007) Glutamine inhibits over-expression of pro-inflammatory genes and down-regulates the nuclear factor kappaB pathway in an experimental model of colitis in the rat. Toxicology 236: 217–226. - PubMed
-
- Kretzmann NA, Fillmann H, Mauriz JL, Marroni CA, Marroni N, et al. (2008) Effects of glutamine on proinflammatory gene expression and activation of nuclear factor kappa B and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm Bowel Dis 14: 1504–1513. - PubMed
-
- Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

